CuSO4 is an inorganic compound formed as a result of sulphur and copper, and HCl is one of the most powerful acids. Let us go over how HCl and CuSO4 react in more detail.
Copper sulphate hydrates as CuSO4.nH2O, where n can range from 1 to 7. In both agricultural and non-agricultural environments, it is used as a fungicide, algaecide, and herbicide. HCl is found in the digestive systems of most animal species, including humans, as a component of gastric acid.
In the following section of the article, let us discuss the mechanism of the reaction between HCl and CuSO4, the reaction enthalpy, the type of reaction, product formation, and so on.
What is the product of HCl and CUSO4?
When concentrated HCl is added to a much-diluted solution of CuSO4, the pale blue solution gradually turns greenish-yellow due to the formation of a copper chloride complex (tetrachlorocuprate (II)).
CuSO4 + 2HCl → CuCl2 + H2SO4
[Cu(H2O)6]2+ + 4Cl– → [CuCl4]2- + 6H2O
Pale blue yellow
What type of reaction is HCl + CuSO4?
The reaction HCl + CuSO4 is a double displacement reaction. The following general scheme represents this reaction:
- AK + MD → AD + KM
- CuSO4 + 2HCl → CuCl2 + H2SO4
- The bond between the reacting species (CuSO4 and HCl) can be ionic or covalent.
- When the ligand or ion exchange occurs in the solid state of the reactant (CuSO4 and HCl), the term double decomposition is used.
How to balance HCl + CuSO4?
The Cl + CuSO4 reaction equation can be balanced by doing the following:
- Step 1: To represent the unknown coefficients, label each compound (reactant or product) in the equation with an alphabet.
- A HCl + B CuSO4 → C H2SO4 + D CuCl2
- Step 2: Create an equation for each element.
- H → A=2C, Cl →A=2D, Cu→ 2B=D, S→B=C, O→B=C
- Step 3: Determine the values of all coefficients and variables using Gaussian elimination.
- Simplify the result to obtain the lowest, whole integer values.
- A=2, B=1, C=1, D=1
- As a result, the overall balanced equation for the preceding reaction is,
- 2HCl + CuSO4 → H2SO4 + CuCl2
HCl + CuSO4 titration
Titration of HCl + CuSO4 is not possible for these reasons.
- HCl is highly acidic.
- CuSO4 is a solid that is white or off-white in color. In the liquid state it contains a strong acid of (H2SO4) and a weak base [Cu(OH)2] salt. As a result, its solution has a pH of 7 ie, highly acidic.
- Two acidic solutions cannot be used for titration.
HCL and CuSO4 intermolecular forces
In Cl + CuSO4 electrostatic forces are the only possible intermolecular forces of attraction for compounds because of the presence of charged species.
HCl + CuSO4 reaction enthalpy
The enthalpy of Cl + CuSO4 reaction (△H) of CuSO4 (0.5g) in water (25 ml) is -10.66g Kj/mol.
Is HCl + CuSO4 a buffer solution?
The reaction HCl + CuSO4 does not produce a buffer solution because, CuSO4 is not a basic salt.
Is HCl + CuSO4 a complete reaction?
HCl + CuSO4 is a complete reaction since a very dilute solution of CuSO4 is treated with concentrated HCl, results in the formation of a copper chloride complex.
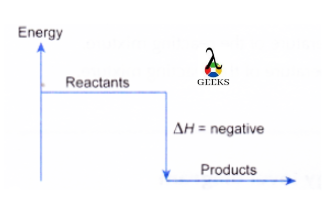
Is HCl + CuSO4 an exothermic or endothermic reaction?
The Cl + CuSO4 reaction is highly exothermic as per thermodynamics because the reaction has a negative enthalpy change. The diagram below shows an energy profile diagram for an exothermic reaction ( the arrow shows the change in energy and this arrow always goes downwards in an exothermic reaction).
Is HCl + CuSO4 a redox reaction?
The reaction HCl + CuSO4 is not redox. Because a complex compound is formed in this reaction, it is a complex formation reaction.
Is HCL + CUSO4 a precipitation reaction?
The reaction HCl + CuSO4 is not a precipitation reaction, it is a complex formation reaction.
Is HCl + CuSO4 reversible or irreversible reaction?
The HCl + CuSO4 is a reversible reaction. This means that anhydrous copper sulphate will be converted back into the blue hydrated form as soon as it comes into contact with water.
How to balance CuSO4 + HCl + Al = AlCl + Cu + H2SO4?
The Cl + CuSO4 + Al reaction equation can be balanced by doing the following:
- Step 1: Label each compound (reactant or product) in the equation with an alphabet to represent the unknown coefficients.
- A CuSO4 + B HCl + C Al →D AlCl + F Cu + G H2SO4
- Step 2: Create an equation for each element (Cu, S, O, H, Cl, Al) where each term represents the number of atoms of the element in each reactant or product.
Cu: 1A=1F, S: 1A = 1G, O: 4A = 4G, H: 1B = 2G, Cl: 1B= 1D, Al: 1C = 1D
- Step 3: Using Gaussian elimination, determine the values of all coefficients and variables.
- A=1, B=2, C=2, D=2, F=1, G=1
- As a result, the overall balanced equation for the preceding reaction is,
- CuSO4 + 2HCl + 2Al → 2AlCl + Cu + H2SO4
How to balance Zn + CuSO4 + HCl = Cu + H2SO4 + ZnCl?
The Cl + CuSO4 + Zn reaction equation can be balanced by doing the following:
• Step 1: Use an alphabet to represent the unknown coefficients in each compound (reactant or product) in the equation.
- A Zn + B CuSO4 + C HCl → D Cu + F H2SO4 + G ZnCl
- Step 2: For each element (Zn, Cu, S, O, H, Cl) write an equation in which each term represents the number of atoms of the element in each reactant or product.
- Zn: 1A=1G, Cu: 1B = 1D, S:1B = 1F, O: 4B= 4F, H: 1C=2F, Cl:1C =1G
- Step 3: Determine the values of all coefficients and variables using Gaussian elimination.
- A=2, B=1, C=2, D=1, F=1, G=2
- As a result, the overall balanced equation for the previous reaction is as follows:
- 2Zn + CuSO4 + 2HCl → Cu + H2SO4 + 2ZnCl
How to balance CuSO4 + Fe + HCl = FeSO4 + CuCl + H2?
HCl + CuSO4 + Fe reaction the equation can be balanced:
• Step 1: Write the unknown coefficients in each compound (reactant or product) in the equation using an alphabet.
- A CuSO4 + B Fe + C HCl → D FeSO4 + F CuCl + G H2
- Step 2: For each element (Cu, S, O, Fe, H, Cl), write an equation with each term representing the number of atoms of the element.
- Cu:1A=1F, S:1A=1D, O:4A=4D, Fe:1B=1D, Cl:1C1F, H:1C=2G
- Step 3: Determine the values of all coefficients and variables using Gaussian elimination.
- A=2, B=2, C=2, D=2, F=2, G=1
- As a result, the overall balanced equation for the preceding reaction is:
- 2CuSO4 + 2Fe + 2HCl → 2FeSO4 + 2CuCl + H2
Conclusion
When concentrated HCl is added to a much diluted CuSO4 solution, the copper chloride complex (tetrachlorocuprate (II)) is formed. The HCl + CuSO4 reaction is a reversible double displacement reaction.
Read more facts on HCl:

Hi … I’m Saina Naushad. I completed my Masters in science with a specialization in Chemistry. I worked with advanced research techniques during my science studies and possessed deep knowledge and expertise in different chemistry topics. I want to help learners better understand advanced Chemistry concepts by sharing my knowledge and skills. please reach out to me on LinkedIn.