Hafnium is a tetravalent transition element. It is silvery-gray in colour. Let us discuss chemical properties of Hafnium below.
Hafnium has hexagonal close-packed structure. Hafnium is an amphoteric oxide. It is ductile. It resists corrosion.
Let us discuss the atomic number, atomic weight, ionization energy etc. of Hf below.
Hafnium symbol
The symbol of Hafnium is Hf.
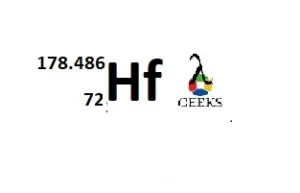
Hafnium group in periodic table
Hafnium belongs to group 4 (Titanium family) of the periodic table.
Hafnium period in periodic table
Hafnium belongs to period 6 of the periodic table.
Hafnium block in periodic table
Hafnium exists in the d-block of the periodic table.
Hafnium atomic number
The atomic number of Hafnium is 72.
Hafnium atomic weight
The atomic weight of the Hafnium is 178.486.
Hafnium Electronegativity according to Pauling
The electronegativity of Hafnium is 1.3 on the Pauling scale.
Hafnium Atomic Density
The atomic density of hafnium is 13.31 g/cm3
Hafnium melting point
The melting point of Hafnium is 2233 oC or 2506 K.
Hafnium boiling point
The boiling point of Hafnium is 4600 oC or 4873 K.
Hafnium Van der waals radius
The Van der waals radius of Hafnium is 225 pm.
Hafnium ionic radius
The ionic radius of Hafnium(4+) is 78 pm
Hafnium isotopes
Isotopes can be stable and unstable. Let us explore the isotopes of Hf.
| Isotope | Abundance | Half-life |
| 174Hf | 0.16% | 2*1015 years |
| 176Hf | 5.26% | stable |
| 177Hf | 18.60% | stable |
| 178Hf | 27.28% | Stable |
| 179Hf | 13.62% | stable |
| 180Hf | 35.08% | stable |
Hafnium electronic shell
The total number of electronic shells in an element are equal to the period number. Let us discuss the electronic shells in Hf.
The total number of electronic shells in Hafnium is 6.
Hafnium energy of first ionization
The first ionization energy of Hafnium is 658.5 kJ/mol.
Hafnium energy of second ionization
The second ionization energy of Hafnium is 1440 kJ/mol.
Hafnium energy of third ionization
The third ionization energy of Hafnium is 2250 kJ/mol.
Hafnium oxidation states
The oxidation states of Hafnium are -2, 0, +1, +2, +3, +4.
Hafnium electronic configurations
The Electronic configuration of Hafnium is [Xe] 4f14 5d2 6s2.
Hafnium CAS number
The CAS number of Hafnium is 7440-58-6.
Hafnium ChemSpider ID
The ChemSpider ID of Hafnium is 22422.
Hafnium allotropic forms
Hafnium exists in two allotropic forms. Let us discuss the allotropic forms of Hafnium below.
Hafnium exists in two allotropic forms –
- α-hafnium with symbol αHf
- β-hafnium with symbol βHf.
Hafnium chemical classification
Hf is a transition element because it contains an incompletely filled penultimate d orbital.
Hafnium state at room temperature
Hafnium exists in solid state at room temperature.
Is Hafnium paramagnetic?
Paramagnetic substances are weakly attracted in a magnetic field. Let us find out if Hf is magnetic or not.
Hafnium is paramagnetic due to the presence of unpaired electrons
Conclusion:
In a nutshell, Hafnium is paramagnetic. It is ductile. It is resistant to corrosion. It exists in two allotropic forms – αHf and βHf. The most oxidation stable state of Hf is +4.

Hi…I am Sonali Jham. I have done my Post-Graduation in Chemistry and also completed B. Ed. I am a Teacher and a Dietician by profession.
My hobby is reading and painting.
Let’s connect through LinkedIn-