Ferric oxide (Fe2O3) is a transition metal oxide, and it reacts with weak sulfurous acid. Let us explore the products obtained from the reaction of Fe2O3 + H2SO3.
Fe2O3 is an amphoteric oxide having both acidic and basic character. Sulfurous acid (H2SO3) is a weak acid, and both of them undergo a neutralization reaction at ambient temperature and pressure.
This article focuses on the products, balancing method, reaction enthalpy, and reversibility with some more relevant topics of the reaction between H2SO3 and Fe2O3 in detail.
What is the product of H2SO3 and Fe2O3?
Ferric sulfite [Fe2(SO3)3] along with water (H2O) are obtained as the products of the reaction between sulfurous acid (H2SO3) and ferric oxide (Fe2O3).
Fe2O3 (aq) + 3H2SO3 (aq) = Fe2(SO3)3 (aq) + 3H2O (l)
What type of reaction is H2SO3 + Fe2O3?
The chemical reaction, H2SO3 + Fe2O3 is one type of-
- Acid-base reaction or neutralization reaction
- Irreversible reaction
- Exothermic reaction
- Double-displacement reaction
How to balance H2SO3 + Fe2O3?
The following steps should be followed to balance a chemical reaction-
- Write the unbalanced chemical equation first using a right arrow sign to signify that the reaction is not balanced yet. Fe2O3 (aq) + H2SO3 (aq) → Fe2(SO3)3 (aq) + H2O (l)
- The mole numbers of each of the reacting elements should be determined on both the reactant and product sides.
| Elements | Mole numbers on the reactant side | Mole numbers on the product side |
| Fe | 2 | 2 |
| S | 1 | 3 |
| O | 6 | 10 |
| H | 2 | 2 |
- To balance both sides (reactant and product) we have to multiply 3 with the mole number of H2SO3 on the reactant side and 3 with the mole number of H2O on the product side.
- Therefore, the final and the balanced equation will be – Fe2O3 (aq) + 3H2SO3 (aq) = Fe2(SO3)3 (aq) + 3H2O (l)
H2SO3 + Fe2O3 Net Ionic Equation
The net ionic equation of the reaction H2SO3 + Fe2O3 is-
2Fe3+ (aq) + 3O2- (aq) + 6H+ + 3SO32- (aq) = 2Fe3+(aq) + 3SO32- (aq) + 3H2O (l)
H2SO3 + Fe2O3 Conjugate Pairs
The conjugate pairs (pair of compounds differ by one proton) of H2SO3 + Fe2O3 is –
- The conjugate pair of H2SO3 is HSO3–
- The conjugate pair of H2O is OH–
- There is no existence of conjugate pairs of ferric oxide as well as ferric sulfite.
H2SO3 + Fe2O3 Titration
The acid-base titration of H2SO3 + Fe2O3 cannot be performed because it is the reaction between a weak acid and an amphoteric oxide (a weak base). In this type of titration, the detection of the equivalence point is not possible as there is no sharp change in the titration graph of the weak acid and the weak base.
H2SO3 + Fe2O3 Intermolecular Forces
The intermolecular forces present in the reaction of H2SO3 + Fe2O3 is-
- Fe2O3 and H2SO3 is ionic compound on the reactant side. Therefore, the Columbic attraction force is working between ferric ion (Fe3+) and oxide ion (O2-) in the lattice of ferric oxide and hydrogen ion and sulfite ion (SO32-) in H2SO3. These two ions are attached by this electrostatic attraction force
- H2O is a covalent compound. Thus, dipole-dipole force and London dispersion forces are present in sulfurous acid molecules. Besides that, it can also form strong intermolecular hydrogen bonding with other H2SO3 molecules.
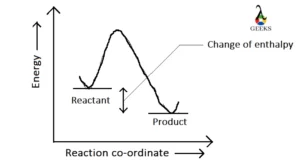
H2SO3 + Fe2O3 Reaction Enthalpy
The enthalpy of the reaction H2SO3 + Fe2O3 are written below-
| Chemical compound | Formation enthalpy |
| H2SO3 | -552 KJ/mol (gas) -600.45 KJ/mol (liquid) |
| Fe2O3 | -824.2 KJ/mol |
| H2O | -286 KJ/mol |
Is H2SO3 + Fe2O3 a buffer solution?
H2SO3 + Fe2O3 is not a buffer solution because neither it is a mixture of a weak acid and its conjugate base (CH3COOH and CH3COONa, acidic buffer) nor a weak base and its conjugate acid (NH4OH and NH4Cl, basic buffer). H2SO3 is a weak acid and Fe2O3 is an amphoteric oxide that acts as a weak base.
Is H2SO3 + Fe2O3 a complete reaction?
H2SO3 + Fe2O3 cannot be a complete reaction because it is only the reactants written here. It will be considered a complete reaction if the reactants along with the products, ferric sulfite [Fe2(SO3)3] and water (H2O), are written here. Therefore, the complete reaction will be –
Fe2O3 (aq) + 3H2SO3 (aq) = Fe2(SO3)3 (aq) + 3H2O (l)
Is H2SO3 + Fe2O3 an exothermic or endothermic reaction?
H2SO3 + Fe2O3 is an exothermic reaction because the change of enthalpy is negative. In an exothermic reaction, heat is absorbed on the product side and generated on the reactant side and the amount of heat absorbed on the product side is always greater than the amount of heat generated on the reactant side.
Is H2SO3 + Fe2O3 a redox reaction?
H2SO3 + Fe2O3 is not a redox reaction because this reaction does not involve any electron transfer. Therefore, no change of oxidation state is taking place. Reacting elements (Fe, S, O and H) do not change their oxidation state from the reactant side to the product side.
| Elements | Oxidation state on the reactant side | Oxidation state on the product side |
| Fe | +3 | +3 |
| H | +1 | +1 |
| S | +4 | +4 |
| O | -2 | -2 |
Is H2SO3 + Fe2O3 a precipitation reaction?
The chemical reaction, H2SO3 + Fe2O3 is not a precipitation reaction because no precipitation of any compound appears on the product side. ferric sulfite [Fe2(SO3)3] is not obtained as a precipitate after the completion of the reaction and it becomes soluble in water.
Is H2SO3 + Fe2O3 a reversible or irreversible reaction?
The reaction, H2SO3 + Fe2O3 is an irreversible reaction due to obtaining more stable products. It is a neutralization reaction and all the neutralization reactions become irreversible due to the formation of more stable salt and water than its corresponding acid and base on the reactant side.
Is H2SO3 + Fe2O3 displacement reaction?
The reaction, H2SO3 + Fe2O3 is a double-displacement reaction. In this reaction, iron is replaced by hydrogen from ferric oxide, and hydrogen itself is replaced by iron from sulfurous acid to obtain the products. Due to happening two displacements, it is defined as a double-displacement reaction.
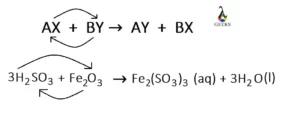
Conclusion
Sulfurous acid has some significant applications in the manufacturing industry of fertilizers, dyes detergents, inorganic salts, etc. Ferric oxide is used as the major feedstock of iron production. It is also used in cosmetics and dental composites.

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202