Hydrogen sulfide is a chalcogen colorless and hydride gas. Let us discuss the physical and chemical properties of hydrogen sulfide.
Hydrogen sulfide is an inflammable gas that exists in the air atmosphere in trace amounts. It is produced from organic substances in absence of oxygen due to the microbial breakdown process. It slowly forms elemental sulfur gas when exposed to air and is slightly soluble in water (3.980 g/litre).
Let us focus on the IUPAC name, radius, magnetism, polarity, reactions, melting, and boiling point of hydrogen sulfide in detail in this article.
H2S IUPAC Name
The IUPAC (International Union of Pure and Applied Chemistry) name of H2S is hydrogen sulfide.
H2S Chemical Formula
Hydrogen sulfide has the chemical formula H2S.
H2S CAS Number
H2S has the CAS registry number (authentic numeric identifier which can contain up to 10 digits) 7783-06-4.
H2S Chem Spider ID
H2S has the ChemSpider (ChemSpider is a free chemical structure database) ID 391.
H2S Chemical Classification
- H2S can be chemically classified as an inorganic gaseous compound.
- H2S can be chemically classified as an acidic compound.
- H2S can be chemically classified as a covalent compound.
- H2S can be chemically classified as chalcogen hydride gas.
H2S Molar Mass
The molar mass of H2S is 34.08 g. This value of the molar mass is obtained from the summation of the molar mass of one sulfur atom and two hydrogen atoms which are 32.065 g/mole and 1.00784 g/mole (molar mass of 1 H atom) respectively.
H2S Color
H2S is a colorless, inflammable, and gaseous compound.
H2S Viscosity
H2S has a viscosity of 0.01166 centipoises at 320 F and 1 atm.
H2S Molar Density
The molar density of H2S is 0.0452 mol/L because it has a density of 1.539 g/L (at 273 K).
H2S Melting Point
H2S has a melting point of -85.50 C or -121.90 F.
H2S Boiling Point
The boiling point of H2S is -59.550 C or -75.190 F.
H2S state at Room Temperature
At room temperature, H2S appears as a colorless gaseous compound because the intermolecular hydrogen bonding in H2S is relatively weak than H2O.
H2S Ionic/Covalent Bond
H2S is a covalently bonded molecule. There are two covalent bonds present in the structure of H2S. All of the two S-H bonds are equivalent and they share their valence electron pairs mutually between them and the bonded electron pairs are slightly shifted towards sulfur due to its greater electronegativity.
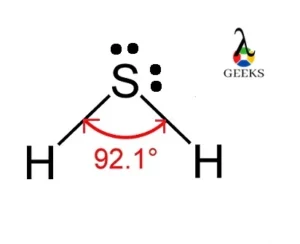
H2S Covalent Radius
The ionic radius of the sulfur (S2-) is 186 pm and the atomic radius of hydrogen is 53 pm.
H2S Electron Configurations
Electron configuration shows the arrangement of the electrons in different energy states, known as electronic shells. Let us explain the electron configuration of H2S in detail.
H2S has the electron configuration [Ne] 3s2 3p4 and 1s1.
H2S Oxidation State
H2S is in the oxidation state of 0 because it is a neutral compound. Sulfur is in -2 (S2-) and each of the two hydrogen atoms is in a +1 (H+) oxidation state.
H2S Acidity/Alkaline
H2S is an acidic (Bronsted as well as lewis acid) gaseous compound. It is a weak acid with an acid dissociation constant (Ka) of 10-7 or pka = 7. H2S = 2H+ + S2-.
Is H2S odorless?
H2S is not an odorless compound because it has a pungent “rotten egg” odor at low concentrations.
Is H2S paramagnetic?
The presence or absence of unpaired electrons which are attracted by the weak magnetic field determines the magnetic behavior of a compound. Let us check the magnetism of H2S.
H2S is not a paramagnetic but diamagnetic compound because there are no unpaired electrons present in H2S. Sulfur in H2S is sp3 hybridized and all the electrons of sulfur as well as the two hydrogen atoms are paired up in those four sp3 hybrid orbitals.
H2S Hydrates
H2S forms gas hydrates with water molecules at low temperatures and high pressure. Stable hydrates are formed when H2S gas molecules are trapped in the cage-like structure developed by the water molecules.
H2S Crystal Structure
The colorless crystal of H2S has a structure of fluorite with the space group of cubic Fm3m and point group C2v. In the crystal, each H+ is bonded with four S2- to form a mixture of corner and edge-sharing HS4 tetrahedra. The lattice parameters are a= b= c = 4.59 A0, and α = β = γ = 900.
H2S Polarity and Conductivity
- Hydrogen sulfide is a polar molecule with a dipole moment of 0.97 Debye. This chemical polarity of H2S arises because of its bent shape. Due to having this shape the bond dipole of two S-H bonds cannot be canceled out by each other.
- H2S is a metallic conductor of electricity above 90 GPa (gigapascal). When it is cooled down below its critical temperature, it exhibits superconductivity.
H2S Reaction with Acid
Though H2S is weakly acidic, it reacts with strong sulfuric acid (H2SO4) and forms elemental sulfur, sulfur dioxide (SO2), and H2O.
H2S + H2SO4 = S + SO2 + 2H2O
H2S Reaction with Base
H2S reacts with a strong base, sodium hydroxide (NaOH) in two manners, and two different products are also obtained.
- H2S + NaOH (excess) = NaS (sodium sulfide) + H2O.
- H2S (excess) + NaOH = NaHS (sodium bisulfide) + H2O.
H2S Reaction with Oxide
H2S undergoes a redox reaction with an oxygen molecule and forms elemental sulfur with water. In this reaction, H2S is oxidized and oxygen is reduced.
2H2S + O2 = 2S + 2H2O
H2S Reaction with Metal
H2S reacts with different metal ions and forms the precipitate of metal sulfide.
- Cu2+ + H2S = CuS (ppt) + 2H+
- Hg2+ + H2S = HgS (ppt) + 2H+
- Pb2+ + H2S = PbS (ppt) + 2H+
- 2Sb3+ + 3H2S = Sb2S3 (ppt) + 6H+
Conclusion
Though hydrogen sulfide is not good for animal health it is used to produce sulfuric acid (H2SO4) and elemental sulfur (S), inorganic sulfides, etc. These sulfides are applied in the leather, pharmaceutical, and pesticide industry. It is also used to form heavy water (D2O) for nuclear power plants.

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202