Germanium is the metalloid present in the periodic table. Let us discuss the electronic configuration of the germanium element.
Germanium has an electronic configuration of [Ar] 3d10 4s2 4p2. It is a p-block element with the atomic symbol ‘Ge’ with an atomic mass of 72.630 u. It has an atomic number of 32; thus, the electrons are arranged in atomic orbitals with different energies to attain an electronic configuration.
In this article, we will be learning all about Ge’s ground state and excited state electronic configuration, along with its ground state orbital diagram and much more.
How to Write Germanium Electron Configuration
The electronic configuration of Germanium is written as [Ar] 3d10 4s2 4p2.
- It is due to the presence of 32 electrons. These electrons are filled in the orbitals as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
- The subshells are first represented by the respective notations of the letter symbol.
- The electrons are filled in the lower energy orbitals first.
- Since 1s2 2s2 2p6 3s2 3p6 is argon’s electronic configuration, a noble gas. It can be abbreviated as Ar.
Germanium Electron Configuration Diagram
The electronic configuration of germanium is [Ar] 3d10 4s2 4p2. It contains 2, 8, 18, and 4 electrons in its shells. The electrons are filled according to the formula: 2n2 where n= 1, 2, 3… n=1 for K, 2 for L, 3 for M, and so on. The electronic configuration of germanium is given below.

Germanium Electron Configuration Notation
The electronic configuration of Germanium is 1s2 2s2 2px2 2py2 2pz2 3s2 3px2 3py2 3pz2 3d10 4s2 4px1 4py1.
Germanium Unabbreviated Electron Configuration
The unabbreviated electronic configuration of Germanium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
Ground State Germanium Electron Configuration
The ground state electronic configuration of Germanium is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 where electrons are filled in the increasing order of energies: 1s, 2s, 2p, 3s, 3p, 4s, 3d..
Excited State of Germanium Electron Configuration
The excited state electronic configuration of Germanium is 1s2 2s2 2p6 3s2 3p6 3d10 4s1 4px1 4py1 4pz1 as shown in the image below.

Ground State Germanium Orbital Diagram
The ground state germanium orbital diagram as per the Pauli exclusion principle is given below.
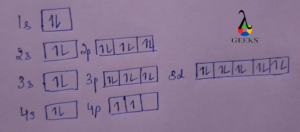
Conclusion
Germanium consists of 32 electrons. The ground electronic configuration can be written as 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 or [Ar] 3d10 4s2 4p2. An electron from a 4s orbital gets excited to a 4p orbital to attain an excited state electronic configuration which is written as 1s2 2s2 2p6 3s2 3p6 3d10 4s1 4px1 4py1 4pz1.
Also Read:
- Dysprosium electron configuration
- Promethium electron configuration
- Ag electron configuration
- Tellurium electron configuration
- Ytterbium electron configuration
- Indium electron configuration
- Polonium electron configuration
- Gallium electron configuration
- Silicon electron configuration
- Strontium electron configuration
Hi, I am Tanisha Singhal. I have completed my post-graduation in Chemistry from the Indian Institute of Technology, Mandi. I have been working as an SME for Chemistry. Here at Lambda Geeks, my articles are aimed at bringing quality chemistry topics in the most effective and straightforward way.