This article contains the enantiomers example, classification, and detailed facts
Enantiomers are configurational isomers, they are formed via bond breaking and making. Enantiomers are mirror images of each other but they are non-superposed to each other.
enantiomers examples are listed below
- 2-bromobutane
- Butan-2-amine
- 2-bromobutan-1-ol
- 2-methylheptan-3-ol
- 3,3-dimethylhexane-1,2-diol
- Methyl-4-amino-3-hydroxy-2,2-dimethylbutanolate
- Hepta-1,5-dien-3-ol
- (1-ethoxyethoxy)cyclohexane
- 1-ethoxy-N-ethylethanamine
- 3-aminopropane-1,2-diol
- 3,4-dihydroxy-4-methylpentane-2-one
- 5-hydroxy-6-methyl-1-phenylheptane-2-one
- 1-(cyclopentyl(methoxy)methyl)cyclopent-1-ene
- 4-methyloctane
- 4-amino-2,7-dimethyloct-1-en-3-ol
- 1-aminohexane-2,3-diol
- But-3-en-2-amine
- 3-methoxy-1-phenylprop-2-en-1-ol
- 3-amino-7-hydroxy-2,6-dimethylheptanal
- 6-methyloctan-2-ol
- 3-chlorocyclohexanol
- 2-methylcyclohexanone
- 1-bromo-2-chlorocyclohexane
- 1-bromo-1-fluoroethane
- Glucose
- Phenylalanine
- 1-bromo-2-chloro-2-fluoro-1-iodoethene
- (2-methylbut-1-en-1-yl)benzene
- (2R,3R)-2,3,4-trihydroxybutanal
- (2R,3S)-2,3,4-trihydroxybutanal
- Acetaldehyde Oxime
Now we discuss the few enantiomers examples and their detailed fact in the following list.
Enantiomers are named according to the position of the functional group in the molecules.
enantiomers examples
- R/S
- D/L
- E/Z
- CIS/TRANS
- SYN/ANTI
- THREO/ERYTHRO
R/S
In 1951 Cahn and Ingold first introduced a nomenclature system for the three-dimensional structure.
After that Cahn, Ingold and Prelog elaborated this system in 1966, and the system is known as CIP nomenclature after the name of the authors. According to this system, the configuration of a molecule is named either R( from Rectus, in Latin the meaning is right) or S (from Sinister, Latin for left).
R and S are always enantiomers to each other.
In this type of Fischer projection,
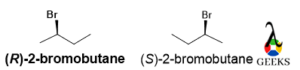
2-bromobutane
The rotation is clockwise and the least priority group belongs below the plane so the configuration is R , and the mirror image the rotation will be anticlockwise so the configuration is S. This is an enantiomers examples.
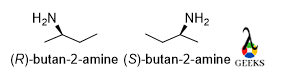
Butan-2-amine
The rotation is clockwise and the least priority group belongs to above the plane so the configuration is S but in the mirror image rotation will be anticlockwise , so the configuration of mirror image is R.
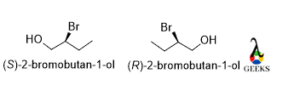
2-bromobutan-1-ol
The rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S but in the enantiomer the rotation will be clockwise so the configuration is R.
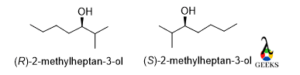
2-methylheptan-3-ol
The rotation will be clockwise and least priority group lies below the plane so the configuration is R but in the mirror image the rotation will be anticlockwise , so the configuration is S.
3,3-dimethylhexane-1,2-diol
The rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S but in the enantiomers the rotation is opposite and the configuration is R. This is an enantiomers examples.

Methyl-4-amino-3-hydroxy-2,2-dimethylbutanolate
The rotation of the molecule is clockwise and the lowest priority group belongs to below the plane so the configuration is R and for the enantiomers the rotation changed to anticlockwise , then the configuration is S.
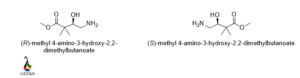
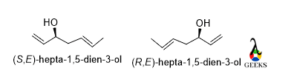
Hepta-1,5-dien-3-ol
Anticlockwise rotation and the lowest priority group belong above the plane so the configuration is S and for the mirror image the rotation will be clockwise and then the configuration will be R.
(1-ethoxyethoxy)cyclohexane
In the molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R. This is an enantiomers examples.

1-ethoxy-N-ethylethanamine
The rotation of the molecule is clockwise and the lowest priority group belongs to below the plane so the configuration is R and for the enantiomers the rotation changed to anticlockwise , then the configuration is S.

3-aminopropane-1,2-diol
In the molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R.

3,4-dihydroxy-4-methylpentane-2-one
In the molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R. This is an enantiomers examples.

5-hydroxy-6-methyl-1-phenylheptane-2-one
The rotation of the molecule is clockwise and the lowest priority group belongs to below the plane so the configuration is R and for the enantiomers, the rotation changed to anticlockwise, then the configuration is S.
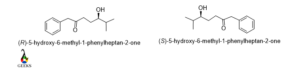
1-(cyclopentyl(methoxy)methyl)cyclopent-1-ene
In the mention molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R. This is an enantiomers examples.

4-methyloctane
In the molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R.
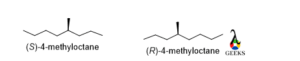
4-amino-2,7-dimethyloct-1-en-3-ol
Here two chiral centers are present so the configuration will be different for two different chiral centres.
For 1st chiral center i.e Carbon number 3 the rotation is anticlockwise and the lowest priority group below the plane, so the configuration is S.
For carbon number 4 the rotation is clockwise and the lowest priority group is below the plane so the configuration is R.
For enantiomers, the rotation will be opposite for two carbon centers so the configuration will be different from the 1st molecule and the configuration is 3R and 4S.

1-aminohexane-2,3-diol
For carbon number 2 the rotation is clockwise and the lowest priority group is below the plane so the configuration is R, and for carbon number 3 again the rotation is clockwise, and the least priority group lies below the plane so the configuration is R.
Again, for enantiomers, the rotation will be opposite for two centers and the configuration will be 2S and 3S. This is an enantiomers examples.

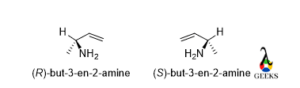
But-3-en-2-amine
The rotation of the molecule is clockwise and the lowest priority group belongs to below the plane so the configuration is R and for the enantiomers, the rotation changed to anticlockwise, then the configuration is S.
3-methoxy-1-phenylprop-2-en-1-ol
In the molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R.
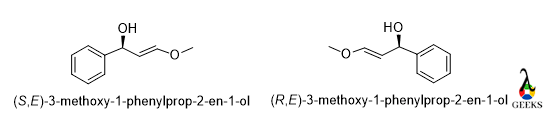
3-amino-7-hydroxy-2,6-dimethylheptanal
The molecule contains three different chiral centers in different environments. So the configuration of these three centers will be different.
First, carbon number 2 the rotation is clockwise and least priority group lies below the plane so the configuration is R. then carbon number 3 the rotation is anticlockwise and the least priority group is below the plane so the configuration is S. now for the last chiral center carbon number 6 the rotation is anticlockwise and least priority group lies to below the plane so the configuration again S.
Now for the enantiomers, all the configurations will be changed due to rotation will be opposite. So in the enantiomer, the configuration is 2S,3R,6R. This is an enantiomers examples.
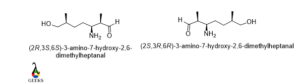
6-methyloctan-2-ol
Here two chiral centers are present in two different environments so the configuration will be different for two different chiral centers.
For the first chiral center carbon number 2 the least priority group is not lying below or above the plane, so an exchange of group is needed here. After the exchange, the rotation is clockwise and the least priority group is below the plane so the configuration is S. For the 6th carbon the rotation is anticlockwise, and the least priority group lies below the plane, so the configuration is R. For enantiomers the configuration is 2R and 6S.
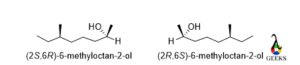
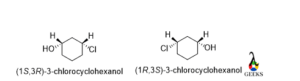
3-chlorocyclohexanol
For 1st carbon, the rotation is clockwise and the least priority group lies above the plane so the configuration is S.
Now for the 3rd carbon the rotation is anticlockwise and the least priority group lies above the plane. So, the configuration is R.
For enantiomer, the configuration is 1R and 3S. This is an enantiomers examples.
2-methylcyclohexanone
The rotation of the molecule is clockwise and the lowest priority group belongs to below the plane so the configuration is R and for the enantiomers, the rotation changed to anticlockwise, then the configuration is S.

1-bromo-2-chlorocyclohexane
For carbon number 1 the rotation is clockwise and the lowest priority group is below the plane so the configuration is R, and for carbon number 2 again the rotation is clockwise, and the least priority group lies below the plane so the configuration is R.
Again, for enantiomers, the rotation will be opposite for two centers and the configuration will be 1S and 2S.

1-bromo-1-fluoroethane
In the below molecule, the rotation is anticlockwise and the lowest priority group belongs below the plane so the configuration is S for the mirror image the rotation will be opposite that is clockwise so the configuration of the enantiomer is R.
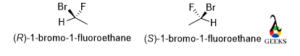
From above examples, we can see that the enantiomer of R is S. they are enantiomers to each other.
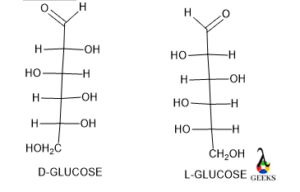
D/L
In 1890 while Ficher was working on sugar and amino acid then he felt that there is a needed a system of nomenclature to identify the configuration of carbohydrates as well as amino acids. Then he established a relative configuration of carbohydrates and amino acids that is D and L.
In the Ficher formula, the main carbon chain lies vertically and the most oxidized carbon lies at the topmost position and then if the hetero group or hetero ligand lies horizontally on the right side then the configuration is D if on the left side then the configuration is L.
Glucose
This is an enantiomers examples.
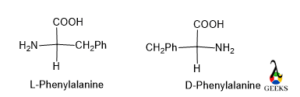
Phenylalanine
E/Z
E/Z nomenclature is for molecule containing double bond (specially for alkene system).
E and Z comes from german word Entagegan means bearing opposite relationship and Zusammem means bearing similar relationship.
1-bromo-2-chloro-2-fluoro-1-iodoethene
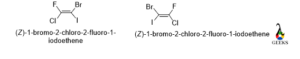
This is an enantiomers examples.
(2-methylbut-1-en-1-yl)benzene
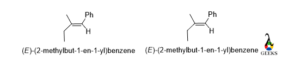
From the above examples, we can conclude that E and Z are not an isomer to each other, because they are relative configurational nomenclature of double bond-containing molecules.
THREO/ERYTHRO
This configuration represents the relative configuration of two adjacent chiral centers.
(2R,3R)-2,3,4-trihydroxybutanal
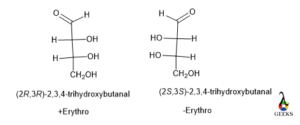
This is an enantiomers examples.
(2S,3R)-2,3,4-trihydroxybutanal
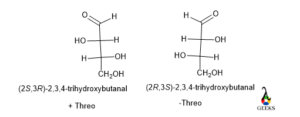
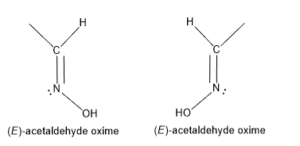
SYN/ANTI
A molecule containing lone pair having this type of isomer, cis-like isomer called syn and a trans-like isomer is called anti.
Acetaldehyde Oxime
FAQ
Are enantiomers optically active ?
Yes. Enantiomers do not contain plane of symmetry so they can optically rotate the plane polarized light.
But in recimic mixture that is the mixture of two enantiomers are optically inactive , cause if r isomer rotates plane polarized light in some angle to right is then s isomers rotates the plane polarized light in same angle to the left side, So, each other cancel out.
What are diastereomers?
Diastereomers are non-mirror images to each other and nonidentical isomers to each other.

Hi……I am Biswarup Chandra Dey, I have completed my Master’s in Chemistry from the Central University of Punjab. My area of specialization is Inorganic Chemistry. Chemistry is not all about reading line by line and memorizing, it is a concept to understand in an easy way and here I am sharing with you the concept about chemistry which I learn because knowledge is worth to share it.