The article discusses about the electrical energy to heat energy conversion that depicts how electrons are charged to lose their energy.
When moving electrons have electrical energy interact with stationary electrons, their energy is converted into chemical energy or light energy. But if their energy is more than the stationary electron’s capacity, the excess of electrical energy is released in the form of heat energy.
The electrical energy is transformed into chemical energy when stationary electrons soak the kinetic energy. It is the process of kinetic energy to potential energy conversion. If more electrons with kinetic energy interact with the chemically bonded stationary electrons with the stored potential energy, it reverses the energy conversion.
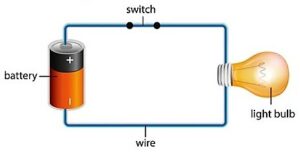
When switching on the electric bulb attached to the battery, the stationary electrons within the battery split their chemical bond. They become free to carry stored potential energy as kinetic energy in the form of electrical energy through the conducting wire. When such electrons contact the tungsten material of the bulb, they interact to generate another energy form.

(credit: shutterstock)
The external electrical energy from the battery breaks the chemical bonds of the electrons within the bulb so that they begin moving rapidly by absorbing external kinetic energy. The temperature is a physical quantity that briefs us on how fast electrons move. Hence, the rapid motion of electrons radiates heat energy from electrical energy.
That’s the reason when the external electric energy flows through the current-carrying conductor, we sense some heat on its surface when we touch it.
Depending on the external electrical energy, electrons hold quicker movement. The quicker the electrons move, the hotter their surface becomes. An object with a higher temperature delivers its heat to other objects at lower temperatures. Therefore, the fast-moving electrons discharge excess energy in the form of light energy.

(credit: shutterstock)
When we add bulb’s light energy and heat energy, we discovered that their sum equals the electrical energy, as per the energy conversion law.
Read about Electrical Energy to Heat Energy Examples.
What Process is Electrical Energy to Heat Energy?
The process of electrical energy to heat energy is electric heating.
Electric heating is the process of yielding heat energy from the chemical elements on the external electric energy passes. The electrical device contains a resistor as a heating element that functions on the Joule heating principle to deliver heat energy, later laboriously employed for commercial purposes.

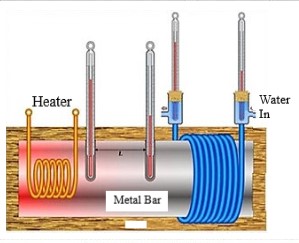
The instant water heater installed in the bathroom is based on the electrical energy to heat energy conversion. Whenever we turn on the heater, we acquire hot water streams. But have you wondered why it takes some time for the water to become hot? The water heater operates on Joule heating or Resistive heating, where heat is created by passing an electric current through the conductor.
Electric heating involves the interactions of moving electrons as a charge carrier with the heating element within the conductor. The electric field developed on the conductor due to potential difference accelerates the stationary electrons. So electrons transmit with kinetic energy towards the direction of the electric field into the conductor.
(credit: shutterstock)
In most electric heaters, a resistor is employed as the heating element. It is two passive terminal components that regulate the flow of electrical energy within the conductor.
The kinetic energy is then transmitted to the fixed electrons when moving electrons hit within the resistor element. The electrons within the resistor are excited to move rapidly due to absorbing kinetic energy. That is when the resistor dissipates the excess energy of its electrons as the heat energy using the Joule heating principle.
The work done (W) of the moving electron into the conductor is given by W = VIt.
where, V is voltage and I is current passing through conductor.
The amount of heat dissipated by the resistor of the conductor is termed as its Power of heating P.
P = W/t = VI
As per Ohm’s law, V = IR,
Therefore, P = I2R, which analogs to Joule’s first law.
Therefore, the principle of Joule heating is derived from Joule’s first law, which states that
“The power of heating (P) of an electrical conductor is proportional to the product of the square of the electric current passes (I) and its resistance(R)”.
Read about Mechanical Energy to Kinetic Energy Conversion
How to Convert Electrical Energy to Heat Energy?
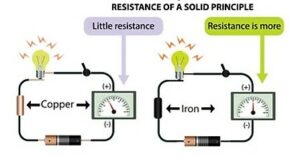
The electrical energy is converted into heat energy due to resistance.
When some quantity of electric current flows through the conducting material, it gets heated. Every conductor has an inbuilt resistance that disperses heat energy when it acquires electrical energy. The phenomenon prevents the conductor from short-circuiting during high electrical energy.
(credit: shutterstock)
Every conductor contains some resistance to maintain the flow of current. We can also manage the current flow by adding an external resistor to the conductor. The desired heat energy value can be obtained from the conductor using Joule heating.
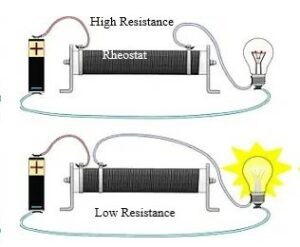
When a current passes through any conductors, its surface becomes hotter. The conductor’s resistance maintains the electrical energy by absorbing and then radiating the exact amount of energy as heat energy. Otherwise, we notice short-circuiting when an immense amount of current passes through the resistance free conductor.
Read more about Electrostatic Charges
Also Read:
- Conservation of mechanical energy examples
- Example of kinetic to sound energy
- Examples of potential energy in your home
- Example of gravitational energy to mechanical energy
- Does mass affect potential energy
- Example of kinetic energy to light energy
- Examples of potential energy
- Does active transport require energy
- Example of kinetic to thermal energy
- What does not affect potential energy

Hello, I’m Manish Naik completed my MSc Physics with Solid-State Electronics as a specialization. I have three years of experience in Article Writing on Physics subject. Writing, which aimed to provide accurate information to all readers, from beginners and experts.
In my leisure time, I love to spend my time in nature or visiting historical places.
Looking forward to connecting you through LinkedIn –