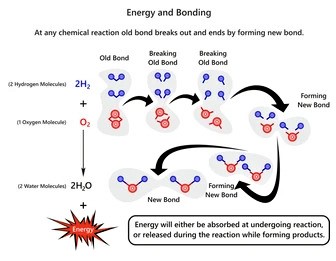
The article discusses about electrical energy to chemical energy conversion that depicts how molecules form chemical bonds due to electric charge transfer.
The flow of electrons carries kinetic energy in the form of electrical energy. The moving electrons induce a chemical reaction when they hit with other material’s chemically bonded electrons. The chemical reactions construct a new molecule that retains energy in chemical energy form.

The electrical to chemical energy transformation illustrates how kinetic energy is changed into potential energy. The kinetic energy allows a stationary charged particle to be in motion. So when the electrons at rest acquire the energy from an external power source, they are excited to move rapidly.
Such moving electrons collide with the other chemical compounds and annihilate their chemically bonded electrons. Hence, the chemical reaction happens between free electrons of the compound, creating a new chemical compound.
(credit: shutterstock)
When the newly bonded stationary electrons store the kinetic energy of moving electrons, it becomes their potential energy. That’s how the new chemical compound holds an equivalent amount of electrical kinetic energy to form chemical potential energy.
Read about Electrical Energy to Chemical Energy Examples
What Process is Electrical Energy to Chemical Energy?
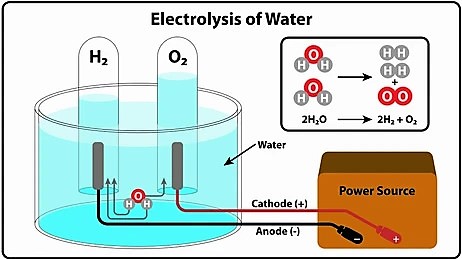
The electrical energy to chemical energy conversion is the electrolysis process.
Electrolysis is the electrochemical process that begins when the electrical energy is delivered from external sources. The outer electrical energy is then accumulated chemically within the chemical compound, permitting us to acquire the electrical energy back out later.
(credit: shutterstock)
Every rechargeable electronic device works on an electrolysis process. When we connect the charger to such a device and switch it on, the electrons within the charger are excited. The excited electrons move with electrical energy towards the rechargeable battery through the wire.
The rechargeable battery includes chemical elements such as electrolytic solution, which permit charge transfer within the battery. So when external energy is delivered to the battery, the chemical bonds within its elements are broken. The electrons get free from chemical bonding and then react to construct new chemical elements by various chemical reactions.
The new chemical elements within the battery then have stationary charge electrons, which store the external electrical energy as chemical potential energy for a more extended period.
Except the charging a storage battery, the example that involves the electrolysis process to convert the electrical to chemical energy is
- Charging Capacitors
- Electroplating
- Cyclotrons
- RF Inductive Heating
- Chip Making
- Electric Welding

Whenever we like to utilize such charged devices, reverse chemical reactions occur. It transforms the chemical potential energy stored within the battery into electrical energy and other kinetic energy forms such as light energy or heat energy.
Read about the Potential Energy to Chemical Energy Examples
How to Convert Electrical Energy to Chemical Energy?
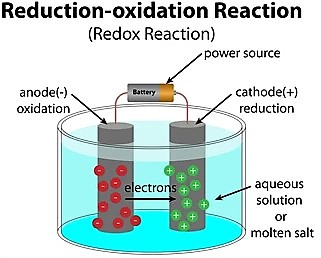
The electricity is converted into chemicals within the electrolytic cell when chemical reactions occur.
The electrolytic cell possesses two chemically dissimilar electrodes. When electrical energy is provided to the cell, a couple of non-spontaneous redox reactions arise at both electrodes, transforming the supplied electrical energy into chemical energy.
(credit: shuttertsock)
The electrochemical electrolytic electrodes are separated by specific distance and dipped into the electrolytic solution, which acts as a solvent. The electrode within a cell connected to the external battery’s positive terminal is termed ‘anode’, whereas the electrode connected to the battery’s negative terminal is termed ‘cathode’.
The positively charged ions, such as cations, get attracted toward the negative electrode. The negatively charged ions, such as anions, get attracted toward the positive electrode.
Suppose both electrodes are immersed into NaCl electrolytic solution and an external power source connected.
When electrical energy is delivered to both the electrodes, moving free electrons are injected into the cathode. Rich in electrons, the cathode attracts the positive ions Na+ within the electrolytic solution. The chemical reaction between Na+ ions and free electrons e– creates a new chemical compound Na, deposited into the cathode electrode.
Na+ + e–→ Na
The half-cell reaction that occurs at the negative cathode is the “reduction redox reaction” that transforms the electrical energy (I) into chemical potential energy.
At the same time as the reduction reaction, the positive anode saturates out its electrons which react with the negative ion Cl within the electrolytic solution. The chemical reaction oxidizes the anode, giving rise to the formation of chlorine Cl2 gas.
2Cl– → Cl2 + 2e–
The half-cell reaction that occurs at the positive anode is the “oxidation redox reaction” that transforms the electrical energy (I) into chemical potential energy.
After both redox reactions, we found that two ions within the electrolytic solution become new chemical compounds.
Therefore, we understood that the electrolysis process created the chemical energy by decomposing both electrodes on the electrical energy passes.
The amount of chemical substance forming during redox reaction is signified by ‘Faraday’s law of electrolysis’, such as the weight of the chemical substance is directly proportional to the quantity of electrical energy passed through the cell.
The anode becomes negative during the discharging process, and the cathode becomes positive because of a reversible chemical reaction to yield electrical energy from stored potential energy. The electrolytic cell is called a ‘galvanic cell’ as it does not demand the external power source to initiate energy conversations.

Read more about Electrostatic Charges
- Work Units: 19 Important Factors Related To It
- Types of Forces : 9 Important Facts You Should Know
- What is Relative Motion : Examples, Exhaustive Concepts, Problems, FAQs
- Inclined Plane: 7 Important Facts You Should Know
- 17 Examples Of Sliding Friction
- Rolling Friction: 15 Important Factors Related To It
Also Read:
- How to estimate energy in an atomic clock
- Can electric potential energy be negative
- Solar energy solar water heater solar pool heater
- How to calculate energy using planck s constant
- Gravitational potential energy to thermal energy
- How to maximize sound energy detection in underwater sonar systems
- How to calculate free energy of a reaction
- How to calculate energy in non linear dynamics
- Why is energy important in electrical engineering
- How to find energy without specific heat

Hello, I’m Manish Naik completed my MSc Physics with Solid-State Electronics as a specialization. I have three years of experience in Article Writing on Physics subject. Writing, which aimed to provide accurate information to all readers, from beginners and experts.
In my leisure time, I love to spend my time in nature or visiting historical places.
Looking forward to connecting you through LinkedIn –