In this article, we are going to see what is double replacement reaction examples in detail.
- Reaction of sodium bicarbonate and acetic acid
- Reaction of Hydrofluoric acid and sodium hydroxide
- Reaction of Barium chloride and sodium sulfate
- Reaction of Silver nitrate and sodium chloride
- Reaction of Potassium iodide and lead nitrate
- Reaction of Barium hydroxide and sulphuric acid
- Reaction of Hydrochloric acid and sodium sulfide
- Reaction of Hydrochloric acid and sodium hydroxide
- Reaction of Potassium carbonate and ammonium iodide
- Reaction of Sodium hydroxide and cesium sulfide
When two complex molecules react with each other resulting in the formation of other complex molecules, in which positive ions and negative ions replace each other, then it is termed as double replacement reaction. ‘Metathesis’ and ‘double displacement reaction’ are other terms referred to as double replacement reactions. As the name suggested both cation and anion show displacement in the reaction.
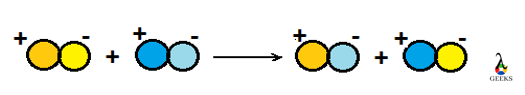
The double replacement reactions are of three types:
Gas formation: The reactions in which the resultant product is in a gas state.
Precipitation: The water-soluble ionic compounds undergo a reaction to form a water-insoluble product then it is termed as a precipitation reaction.
Neutralization: Usually acids and bases show this type of reaction The salt and water are formed as products by the reaction of acid – base.
Also Read On: 15 Coordinate Covalent Bond Examples: Detailed Insight And Facts
Double replacement reaction examples
Reaction of sodium bicarbonate and acetic acid
The sodium bicarbonate reacts with acetic acid it resulting in the formation of carbonic acid sodium acetate and carbonic acid. As positively charged hydrogen, sodium ions and negatively charged carbonic and carboxyl group exchange in the products. Hence, an example of a double replacement reaction.

Reaction of Hydrofluoric acid and sodium hydroxide
The hydrofluoric acid and sodium hydroxide undergo reaction to form sodium fluoride along with water. The cation and anion show replacement with other identically charged ions. It is a neutralization reaction.

Reaction of Barium chloride and sodium sulfate
In the reaction of Barium chloride and sodium sulfate, positively charged Barium and sodium ion replaces each other on the other hand negatively charged chloride and sulfide ions replace each other.

Reaction of Silver nitrate and sodium chloride
The Silver nitrate and sodium chloride undergo a reaction to form sodium nitrate along with silver chloride. The cation and anion show replacement with other identically charged ions. It is a precipitation reaction, as a water-insoluble precipitate of silver chloride formed.

Reaction of Potassium iodide and lead nitrate
The potassium iodide and lead nitrate undergo a chemical reaction and potassium nitrate and lead iodide are formed as products. As positively charged potassium, lead ions and negatively charged nitrate, iodide ions are exchanged in the products.

Reaction of Barium hydroxide and sulphuric acid
The barium hydroxide and sulphuric acid undergo a reaction to form barium sulfate along with water. The cations barium and hydronium ion replace each other as well as anions hydroxide and sulfide shows replacement.

Reaction of Hydrochloric acid and sodium sulfide
The hydrochloric acid reacts with aqueous sodium sulfide it resulting in the formation of hydrogen sulfide gas and sodium chloride. As positively charged hydrogen, sodium ions and negatively charged chloride, sulfide ions exchange in the products.

Reaction of Hydrochloric acid and sodium hydroxide
The salt and water form as a product when acid and base undergo a chemical reaction. The positively charged ions of one molecule form a bond with negatively charged ions of another molecule and vice versa. Sodium chloride and water are formed by the reaction of hydrochloric acid and sodium hydroxide.

Reaction of Potassium carbonate and ammonium iodide
In the reaction of potassium carbonate and ammonium iodide, positively charged potassium and ammonium ion replace each other on the other hand negatively charged carbonium and iodide replace each other.

Reaction of Sodium hydroxide and cesium sulfide
The Sodium hydroxide reacts with aqueous cesium sulfide resulting in the formation of sodium sulfide gas and cesium hydroxide. As positively charged cesium, sodium ions and negatively charged hydroxide, sulfide ions exchange in the products.

Also Read On: 10 Ionic Bond Examples: Explanation And Detailed Facts
Frequently asked questions:
1) Question: What is double replacement reaction?
Answer: A double replacement reaction is defined as,
When two complex molecules react with each other resulting in the formation of other complex molecules, in which positive ions and negative ions replace each other, then it is termed as double replacement reaction. Metathesis and double displacement reaction are other terms that are used for double replacement reaction. As the name suggested both cation and anion show displacement in the reaction.
2) Question: What are the types of double replacement reactions?
Answer: The types of double replacement reactions are,
Gas formation: The reactions in which the resultant product is in a gas state.
Precipitation: The water-soluble ionic compounds undergo a reaction to form a water-insoluble product then it is termed as a precipitation reaction.
Neutralization: Usually acids and bases show this type of reaction. The salt and water are formed as products by the reaction of the acid – base.
3) Question: How do you know if a double replacement reaction will occur?
Answer: To figure out whether a double replacement reaction will occur,
The double replacement reaction occurs when ionic compounds react in aqueous solutions resulting in the formation of other ionic compounds. In this reaction, ions show replacement. By tracking the positions of cations and anions in reactants and products one can find whether the double displacement reaction occurs or not.
4) Question: Can a double displacement reaction takes place when the products are highly soluble?
Answer: The double replacement reaction does not take place.
The double displacement reactions can be performed only in aqueous solutions. It requires compounds having partial solubility which leads to forming ions. So that cations and anions can replace each other. Hence when the products are highly soluble, a double displacement reaction can not take place.
Also Read On: 5+ Double Bond Examples: Detailed Insights And Facts
I am Smruti Bhosale. I am from Mumbai. I have Master’s degree in Inorganic chemistry from Guru Nanak Khalsa College, Mumbai. I always have a passion for writing and to inspire as many willing minds through my words. Chemistry is a subject that is used by everyone in their normal lives.
I want to explain the subject in the most understandable and simplest way possible. I am a creative, hard working person and passionate about learning new things. I like to read books.