Summary
Distilled water density is a critical property in various scientific fields, including chemistry and physics. The density of distilled water can be measured using different techniques and equipment, such as burettes, graduated cylinders, beakers, and pycnometers. These methods aim to determine the mass and volume of distilled water accurately, which are essential for calculating its density. Understanding the physics behind density and its role in related phenomena, such as buoyancy, is crucial for applying this knowledge in practical situations.
Understanding Distilled Water Density
Distilled water is a pure form of water that has been purified through the process of distillation, removing any impurities or dissolved minerals. The density of distilled water is a crucial property that is widely used in various scientific and industrial applications.
Defining Density
Density is a fundamental physical property that describes the mass of a substance per unit volume. It is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³). The density of a substance can be calculated using the formula:
ρ = m / V
Where:
– ρ (rho) is the density of the substance
– m is the mass of the substance
– V is the volume of the substance
Factors Affecting Distilled Water Density
The density of distilled water can be influenced by several factors, including:
-
Temperature: The density of distilled water is highly dependent on temperature. As the temperature increases, the density of distilled water decreases. This is due to the thermal expansion of the water molecules, which causes them to occupy a larger volume.
-
Pressure: The density of distilled water can also be affected by changes in pressure. As the pressure increases, the density of distilled water slightly increases due to the compression of the water molecules.
-
Impurities: The presence of dissolved substances or impurities in the water can affect its density. Distilled water, being a pure form of water, has a well-defined and consistent density compared to water with dissolved solutes.
Measuring Distilled Water Density
There are several methods and techniques used to measure the density of distilled water, each with its own advantages and limitations. Some common methods include:
-
Pycnometry: Pycnometers are specialized glass containers with a known volume that are used to determine the density of liquids. The pycnometer is filled with distilled water, and its mass is measured. The density is then calculated by dividing the mass of the water by the known volume of the pycnometer.
-
Graduated Cylinder or Beaker: The mass of a known volume of distilled water can be measured using a graduated cylinder or beaker. The volume of the water is determined by the markings on the container, and the mass is measured using a balance. The density is then calculated by dividing the mass by the volume.
-
Buoyancy Method: This method involves submerging an object with a known mass and volume in distilled water. The buoyant force acting on the object is measured, and the density of the water is calculated using Archimedes’ principle.
-
Hydrometer: A hydrometer is a device that measures the specific gravity or relative density of a liquid, such as distilled water, by measuring the depth to which the instrument sinks in the liquid.
Distilled Water Density Values
The density of distilled water at standard temperature and pressure (20°C and 1 atm) is approximately 0.998 g/cm³ or 998 kg/m³. However, the exact value can vary slightly depending on the specific conditions and measurement techniques used.
Here are some more detailed values for the density of distilled water at different temperatures:
| Temperature (°C) | Density (g/cm³) |
|---|---|
| 0 | 0.999840 |
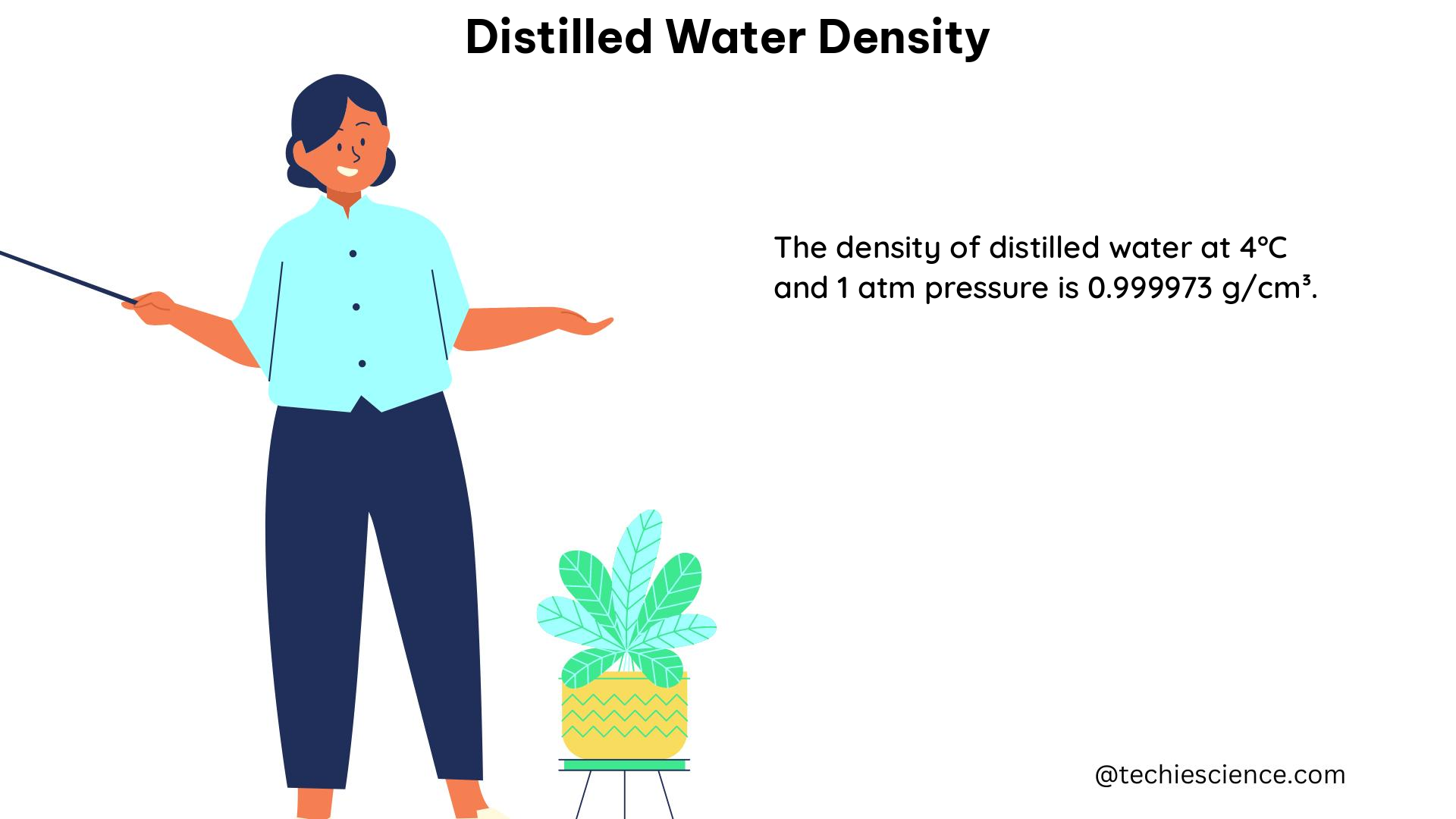
| 4 (maximum) | 0.999973 |
| 10 | 0.999703 |
| 20 | 0.998203 |
| 30 | 0.995650 |
| 40 | 0.992217 |
| 50 | 0.988028 |
| 60 | 0.983133 |
| 70 | 0.977565 |
| 80 | 0.971364 |
| 90 | 0.964565 |
| 100 | 0.958025 |
It’s important to note that these values are approximate and may vary slightly depending on the specific measurement conditions and the purity of the distilled water.
The Importance of Distilled Water Density in Physics
The density of distilled water is a crucial property in various areas of physics, particularly in the study of fluid dynamics and buoyancy.
Fluid Dynamics
The density of distilled water is a fundamental parameter in the study of fluid dynamics, which involves the behavior of liquids and gases in motion. Distilled water, being a pure and well-defined liquid, is often used as a reference fluid in experiments and simulations related to fluid flow, viscosity, and other fluid properties.
Bernoulli’s Principle
Bernoulli’s principle states that as the speed of a fluid increases, the pressure within the fluid decreases. This principle is directly related to the density of the fluid, as it governs the relationship between pressure, velocity, and elevation in a flowing fluid. Understanding the density of distilled water is essential for accurately applying Bernoulli’s principle in various applications, such as the design of airfoils and fluid flow systems.
Navier-Stokes Equations
The Navier-Stokes equations are a set of fundamental equations that describe the motion of fluids, including distilled water. These equations incorporate the density of the fluid as a key parameter, and accurate knowledge of distilled water density is necessary for solving these equations and predicting fluid behavior.
Buoyancy and Archimedes’ Principle
Archimedes’ principle states that the buoyant force on an object submerged in a fluid is equal to the weight of the fluid displaced by the object. The density of the fluid, in this case, distilled water, is a crucial factor in determining the buoyant force and the behavior of objects in the fluid.
Understanding the density of distilled water is essential for accurately calculating the buoyant force and predicting the behavior of objects, such as ships, submarines, and other floating or submerged structures, in water.
Density-Based Separation Techniques
The density of distilled water is also important in various separation techniques, such as centrifugation and density gradient centrifugation, which are used to separate and purify different substances based on their densities.
In these techniques, the density of distilled water is often used as a reference to determine the relative densities of the substances being separated, allowing for efficient and accurate separation processes.
Practical Applications of Distilled Water Density
The density of distilled water has numerous practical applications in various fields, including:
-
Chemistry and Analytical Techniques: Distilled water is commonly used as a reference fluid in analytical techniques, such as titrations, where the density is used to calculate the concentration of solutions.
-
Environmental Studies: The density of distilled water is important in the study of water quality, pollution, and the behavior of aquatic ecosystems, as it affects the transport and distribution of dissolved substances and suspended particles.
-
Engineering and Design: The density of distilled water is a crucial parameter in the design and analysis of water-based systems, such as hydraulic systems, water treatment plants, and cooling systems.
-
Pharmaceutical and Biomedical Applications: Distilled water is widely used in the pharmaceutical industry and biomedical research, where its well-defined density is important for formulations, drug delivery systems, and various analytical procedures.
-
Food and Beverage Industry: The density of distilled water is a reference point for measuring the density of other liquids, such as juices, syrups, and alcoholic beverages, which is important for quality control and product development.
-
Calibration and Standardization: Distilled water is often used as a reference fluid for calibrating and standardizing various measurement instruments, such as hydrometers, densitometers, and other density-related equipment.
Conclusion
The density of distilled water is a fundamental property that plays a crucial role in various scientific and industrial applications. Understanding the factors that affect distilled water density, the methods used to measure it, and its importance in physics and practical applications is essential for researchers, engineers, and professionals working in diverse fields.
By mastering the concepts and techniques related to distilled water density, you can enhance your understanding of fluid dynamics, buoyancy, and other related phenomena, ultimately leading to more accurate and efficient problem-solving in your area of expertise.
Reference:
- Measurements in the Laboratory (Experiment). (n.d.). Retrieved from https://chem.libretexts.org/Ancillary_Materials/Laboratory_Experiments/Wet_Lab_Experiments/General_Chemistry_Labs/Online_Chemistry_Lab_Manual/Chem_9_Experiments/01:Measurements_in_the_Laboratory(Experiment)
- Pycnometer Usage and Density Calculations. (n.d.). Retrieved from https://www.vaia.com/en-us/textbooks/chemistry/chemistry-13-edition/chapter-1/problem-94-a-pycnometer-is-a-device-for-measuring-the-densit/
- Exploring Accuracy and Precision: Calculating Water Density with. (n.d.). Retrieved from https://www.coursesidekick.com/chemistry/1147899

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.