In this article we are discussing about bro4- lewis structure, characteristics and 13 important facts regarding this.
Perbromate (bro4-) is an oxoanion of bromine. Like clo4-, io4- it is difficult to prepare. The molar mass of bro4- ion is 143.901 g/mol. When bro3- ion reacts with fluorine in alkaline medium, bro4- ion is produced. When perbromic acid losses one proton bro4- ion is produced.
Bro4- Lewis Structure Drawing
In the bro4- lewis structure we see that as bromine becomes bigger in size and less electronegative than O, br act as the central atom in the lewis structure of bro4- ion. Bromine has 7 valance electrons out of which 4 electrons take part in sigma bonding with 4 O atoms and forms 3 pi bonds with 3 O atoms.
There is no remaining electrons on br atom which act as lone pair of electron. O atom has 6 electrons in the valance shell out of which 1 is used in making sigma bond and another is used in making pi bond with br atom and remaining 4 electrons present as nonbonding electron pair on O atom.

Bro4- Lewis Structure Resonance
Resonance is an empirical process in which movement of electron pair takes place from one atom to another atom and the structure obtained by this process is called resonating or canonical structure.
Bro4- has 4 resonating structure in which each Br-O bond acquires partial double bond character through the process in which negative charge on O atom is delocalized with the empty ∏* antibonding orbital of Br=O bond.

Bro4- Lewis Structure Shape
According to VSEPR theory the shape of Bro4- is tetrahedral in which central Br atom is tetrahedrally surround by 3 O atoms and 1 O- ion. As lone pair absent in the central Br atom, Bro4- ion possesses true tetrahedral structure. As the shape is tetrahedral, the bond angle occurs in this compound is exactly 109.280.

Bro4- Lewis Structure Formal Charge
The formal charge on any atom or ion can be obtained by the formula which is given below:
Formal Charge (f.c) =V.E- B.E/2- N.E
Where,
V.E= Total No of valance electrons, B= Total bonding electrons, N= Total No of nonbonding electrons.
Hence formal charge on br atom in bro4- ion = 7-14/2-0=0.
Formal charge on each double bonded O atom in bro4- ion =6-4/2-4=0.
Formal charge on single bonded O atom in bro4- ion =6-2/2-6=-1.
Hence formal charge on central br atom is 0.Each double bonded O atom has 0 and single bonded O atom has -1 formal charge making the overall compound is negatively charged.
Bro4- Lewis Structure Angle
In bro4- br uses sp3 hybrid orbitals to form bro4- ion. The structure of bro4- is tetrahedral. As lone pair of electron is not present in bro4- ion the angle is exactly same as that of in real tetrahedral structure. The angle in bro4- ion is 109.280 i.e. the o-br-o bond angle is 109.280.

Bro4- Lewis structure Octet Rule
In the bro4- lewis structure we see that each O atom has 8 valance electrons and fulfill their octet. In bro4- ion br forms 3 br=O bonds and 1 br-O bond making a total of 14 electrons around central br atom.
As br is a congener of 4th period element br atom can increase their octet more than 8 electrons due to presence of vacant d orbital. Hence according to octet rule bro4- is a stable compound.
Bro4- Lewis Structure Lone Pair
The valance electron which does not take part in bonding is defined as lone pair of electrons or nonbonding electrons.
The formula which is used to calculate the lone pair of electron on the given atom is given below:
No of lone pair of electrons = no of valance electrons present on the atom-no of covalent bonds formed by that atom.
In bro4- ion lone pair present on br atom= 7-7=0 i.e. zero lone pair of electrons.
Lone pair of electrons present on each double bonded O atom=6-2=4 i.e. 2 lone pair of electrons.
Lone pair of electrons present on single bonded O- ion= 8-2=6 i.e.3 nonbonding electron pairs.
These lone pairs are shown in the lewis structure of bro4- ion on the given atoms as electron dots.
Bro4- Valance Electrons
At first, to calculate the total valance electron in bro4-, it is important to know the electronic configuration of br atom and O atom. The electronic configuration of br atom is [Ar18] 4s2 3d10 4p5 and from electronic configuration we see that there are 7 electrons in the valance shell of br atom.
The electronic configuration of O atom is [He2] 2s2 2p4 i.e. 6 valance electrons present in O atom.1 negative charge is also present. The total valance electrons in bro4- ion will be equal to the sum of the valance electron of br atom and O atom+1 negative charge i.e. (7*1)+(6*4)+1=32. There are 32 valance electrons in bro4- ion.
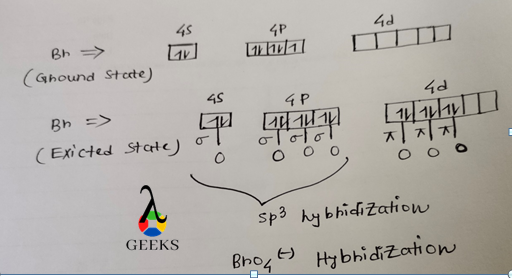
Bro4- Hybridization
Hybridization is the process of mixing of similar energy atomic orbitals to produce an equal number of hybrid orbitals.
The ground state valance shell electronic configuration of br atom is 4s2 4p5. In the ground state of br atom only 1 unpaired electron is present and to make bro4-, 4 unpaired electron is required. In the excited state br send its 1 4s electrons and 2 4p electrons into vacant d orbital making a total of 7 unpaired electrons.
In the next step 4 o atoms give their 1 unpaired electron to form 4 br-o sigma bonds and 4 O atoms give their remaining unpaired electron to form 3 br=O (pi) bonds. In this compound br uses sp3 hybrid orbital to make br-O bonds. According to sp3 hybridization, the geometry of bro4- ion will be tetrahedral.
Bro4- solubility
Bro4- ion is soluble in polar solvents like water, ethanol, methanol etc. When Hbro4 losses 1 proton bro4- ion is formed i.e. bro4- ion is a conjugate base of Hbro4.
Hence, bro4- ion behaves as a base and has a strong tendency to accept proton. When it reacts with water, bro4- ion accepts 1 proton from water to form Hbro4 and Oh- ions making the aqueous medium alkaline.
Is BrO4- Ionic?
Bro4- is an ionic compound. This is because of the fact that it is formed when Hbro4 losses 1 proton. It is an anionic species.
Like ionic species, it has a strong tendency to react with water and also aq. solution of bro4- ion conducts electricity like other ionic compounds does. Like other ionic compounds it has high melting point. These facts suggest that bro4- ion is an ionic compound.
Is Bro4- acidic or basic?
Bro4- ion is a basic compound. It is the conjugate base of Hbro4 acid. Like other bases, it has a strong tendency to accept protons to form acid.
It also has high electron density on it like other bases and donates its electron density towards a proton or a lewis acid. Hence it act as a lewis base and forms adduct with lewis acid.
Is Bro4- polar or nonpolar?
Bro4- is nonpolar in nature. A compound is said to be nonpolar if its dipole moment is equal to zero. In this compound 4 br-O bonds are polar this is due to electronegativity difference between br and O atom.
As O is more electronegative than br atom, br-o bond moment lies towards o atom. As bro4- has real tetrahedral shape 4 br-o bond moments cancel each other and hence dipole moment becomes equals to 0.
By considering the above mentioned facts, bro4- ion is an ionic, covalent compound which has tetrahedral geometry. It is basic in nature and has a tendency to accept proton from any protonic solvent. Bro4- ion is not much stable due large size difference between Br and O atom.
Also Read:
- Ca2 lewis structure
- Oh lewis structure
- Brf2 lewis structure
- Obr2 lewis structure
- Pf2cl3 lewis structure
- Co2 lewis structure
- Sibr4 lewis structure
- Xebr2 lewis structure
- Chf3 lewis structure
- Hso3 lewis structure

Hi….I am Susanta Maity. I have completed my Masters from Vidyasagar university with a specialization in organic chemistry.
I love to write complicated Chemistry concepts in understandable and simple words.
Let’s connect through LinkedIn: