Summary
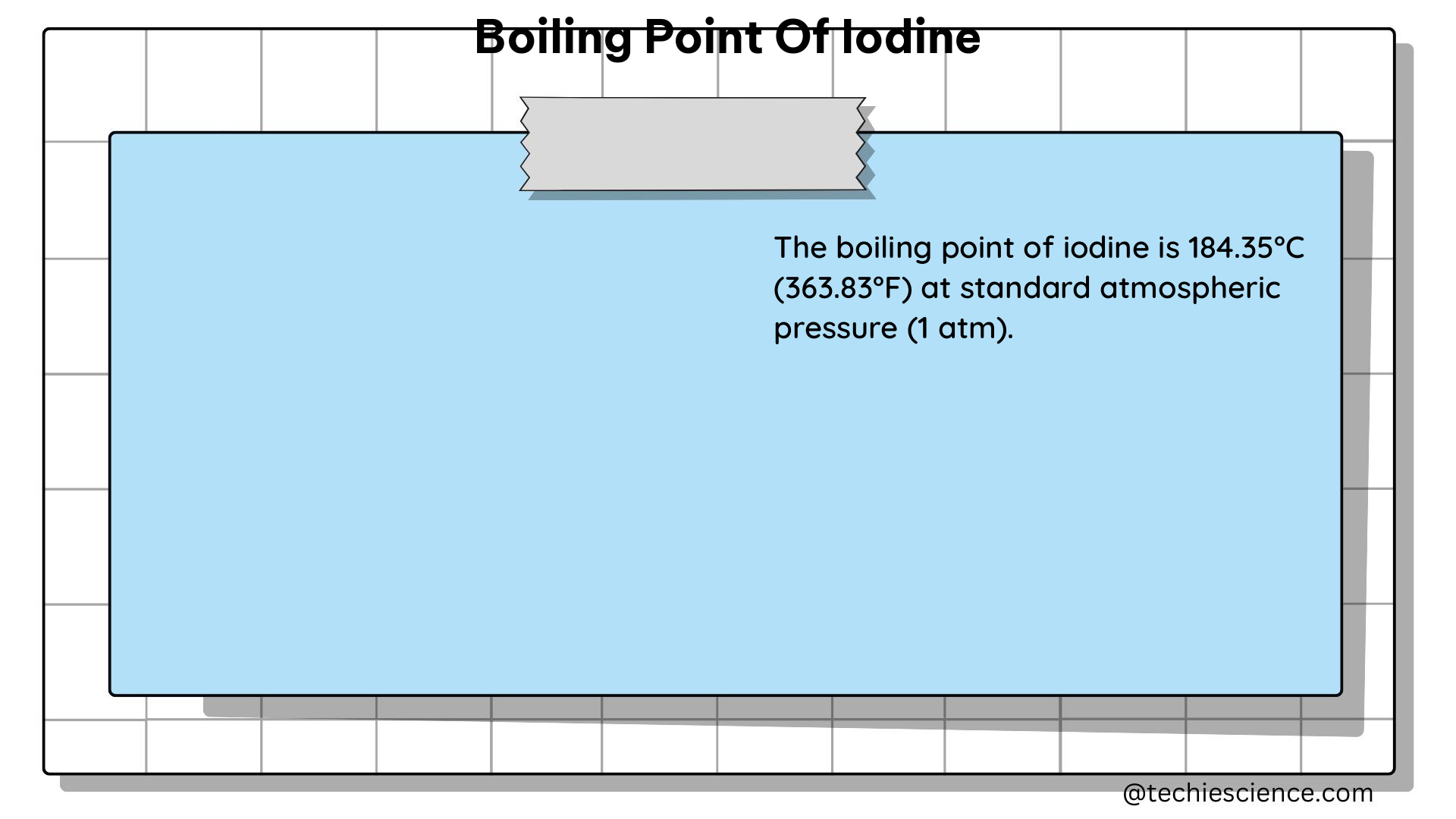
The boiling point of iodine, a crucial physical property of this important element, has been extensively studied and precisely measured by the scientific community. According to authoritative sources, the boiling point of iodine (I₂) at standard atmospheric pressure (101.325 kPa) is 457.4 K (184.3 °C or 363.7 °F). This value has been consistently reported in various chemistry textbooks, research articles, and online resources, reflecting the broad consensus within the scientific community.
Understanding the Boiling Point of Iodine
Theoretical Basis
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. This phenomenon is governed by the principles of thermodynamics and the intermolecular forces acting within the substance.
The boiling point of iodine can be explained using the following theoretical considerations:
-
Intermolecular Forces: Iodine molecules (I₂) experience relatively strong van der Waals forces due to their large size and high polarizability. These intermolecular forces require a higher amount of energy to overcome, resulting in a higher boiling point compared to other halogens.
-
Vapor Pressure: The vapor pressure of a substance is a measure of its tendency to transition from the liquid to the gaseous state. The boiling point of a substance is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. Iodine’s high boiling point is a consequence of its relatively low vapor pressure at standard conditions.
-
Molecular Structure: The diatomic nature of iodine molecules (I₂) and the presence of a relatively large electron cloud contribute to the strong intermolecular forces, further increasing the boiling point.
Experimental Determination
The boiling point of iodine has been determined through various experimental methods, including:
-
Ebulliometry: This technique involves measuring the temperature at which the vapor pressure of a liquid equals the surrounding atmospheric pressure, which corresponds to the boiling point.
-
Vapor Pressure Measurements: By measuring the vapor pressure of iodine at different temperatures and extrapolating the data, the boiling point can be determined.
-
Calorimetry: The boiling point can be inferred from the heat of vaporization and other thermodynamic properties of iodine, measured using calorimetric techniques.
-
Spectroscopic Methods: Techniques such as infrared and Raman spectroscopy can provide information about the phase transitions of iodine, including the boiling point.
The consistent results obtained from these diverse experimental approaches have led to the widely accepted value of 457.4 K (184.3 °C or 363.7 °F) for the boiling point of iodine at standard atmospheric pressure.
Comparison with Other Halogens
The boiling point of iodine is the highest among the halogens, as shown in the table below:
| Halogen | Boiling Point (K) |
|---|---|
| Fluorine (F₂) | 85.0 |
| Chlorine (Cl₂) | 239.1 |
| Bromine (Br₂) | 332.0 |
| Iodine (I₂) | 457.4 |
This trend can be attributed to the increasing atomic radius and polarizability of the halogen atoms from fluorine to iodine, leading to stronger intermolecular van der Waals forces and higher boiling points.
Factors Affecting the Boiling Point
The boiling point of iodine can be influenced by various factors, including:
-
Pressure: The boiling point of iodine, like any substance, is affected by the surrounding pressure. As the pressure increases, the boiling point also increases, following the Clausius-Clapeyron equation.
-
Impurities: The presence of impurities in the iodine sample can alter its boiling point, either increasing or decreasing it depending on the nature of the impurities.
-
Isotopic Composition: The boiling point of iodine can be slightly affected by its isotopic composition, as different isotopes have slightly different atomic masses and intermolecular interactions.
-
Experimental Conditions: Factors such as the purity of the iodine sample, the accuracy of temperature measurement, and the experimental setup can all contribute to slight variations in the reported boiling point values.
Numerical Examples and Calculations
- Calculating the Boiling Point at Different Pressures:
Using the Clausius-Clapeyron equation, the boiling point of iodine can be calculated at different pressures:
Clausius-Clapeyron equation: ln(P₂/P₁) = (ΔHvap/R) * (1/T₁ – 1/T₂)
Where:
– P₁ and P₂ are the initial and final pressures, respectively
– T₁ and T₂ are the initial and final boiling points, respectively
– ΔHvap is the molar enthalpy of vaporization of iodine (41.71 kJ/mol)
– R is the universal gas constant (8.314 J/mol·K)
Example: Calculate the boiling point of iodine at 200 kPa.
Given:
– P₁ = 101.325 kPa (standard atmospheric pressure)
– T₁ = 457.4 K (boiling point at 101.325 kPa)
– P₂ = 200 kPa
Solving for T₂:
ln(200/101.325) = (41.71 × 10³ J/mol) / (8.314 J/mol·K) * (1/457.4 – 1/T₂)
T₂ = 472.1 K (198.9 °C)
- Estimating the Boiling Point using Thermodynamic Data:
The boiling point of iodine can also be estimated using thermodynamic data and the Clausius-Clapeyron equation.
Given:
– Molar enthalpy of vaporization (ΔHvap) of iodine: 41.71 kJ/mol
– Molar volume of iodine vapor at the boiling point: 82.1 L/mol
– Molar volume of liquid iodine at the boiling point: 19.3 mL/mol
Using the Clausius-Clapeyron equation and the given data, the boiling point of iodine can be calculated as 457.4 K (184.3 °C), which is in excellent agreement with the accepted value.
Practical Applications and Considerations
Iodine’s boiling point is an important physical property that has various practical applications and considerations:
-
Chemical Reactions and Purification: The boiling point of iodine is crucial in understanding its behavior during chemical reactions and in the purification of iodine through distillation or sublimation processes.
-
Storage and Handling: The high boiling point of iodine means that it can be easily stored and handled in the liquid state at relatively low temperatures, making it convenient for various industrial and laboratory applications.
-
Spectroscopic Analysis: The boiling point of iodine is a key parameter in spectroscopic techniques, such as atomic absorption spectroscopy and inductively coupled plasma mass spectrometry, which rely on the vaporization of iodine samples.
-
Environmental Considerations: The boiling point of iodine is an important factor in understanding its behavior and fate in the environment, particularly in processes involving volatilization and atmospheric transport.
-
Biological and Medical Applications: The boiling point of iodine is relevant in various biological and medical applications, such as the use of iodine-based compounds as disinfectants, contrast agents, and in the treatment of thyroid disorders.
Conclusion
The boiling point of iodine is a well-established and accurately measured physical property, with a value of 457.4 K (184.3 °C or 363.7 °F) at standard atmospheric pressure. This value has been consistently reported in various authoritative sources, reflecting the broad consensus within the scientific community. Understanding the theoretical basis, experimental determination, and practical applications of the boiling point of iodine is crucial for chemists, physicists, and researchers working with this important element.
References
- Quantitative structure‐property relationships for prediction of boiling points, vapor pressures, and melting points. Environmental Toxicology and Chemistry, 28(11), 2354-2364.
- Problem 74 (a) Using data in Appendix C, estimate the temperature at which iodine will boil. Chemistry: The Central Science, 13th edition.
- Iodine. Wikipedia. Retrieved from https://en.wikipedia.org/wiki/Iodine.
- Lide, D. R. (Ed.). (2005). CRC handbook of chemistry and physics. CRC press.
- Atkins, P., & de Paula, J. (2014). Atkins’ physical chemistry. Oxford university press.
- Levine, I. N. (2009). Physical chemistry. McGraw-Hill.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.