Barium is the fifth element in group IIA in the periodic table and is an alkaline earth metal. Let us study some facts about the element in detail.
The electronegativity of Ba is 0.89 as per the Pauling scale. Barium is present in the 6th period with an atomic mass of 137.24 g/mol. The electronic configuration of Ba is [Xe]6s2 and is silvery in appearance. There are a myriad of applications for barium in oil refineries and the manufacture of bricks and rubber.
Here, we will discuss the trends of electronegativity and ionization energies (I.E.) of barium and will compare them with that of other elements in detail.
Barium and chlorine electronegativity
The electronegativity difference between Barium and chlorine is 2.27. Cl is more electronegative than Ba as,
| Electronegativity of barium | Electronegativity of chlorine | reason |
|---|---|---|
| 0.89 | 3.16 | Being a metal Ba has a very low electron affinity. Cl is present in the 17th group (halogens) and has the highest electron affinity so has high electronegativity. |
Barium and tin electronegativity
Ba and Sn are elements of different groups. Their electronegativity values will differ as,
| Electronegativity of barium | Electronegativity of tin | reason |
|---|---|---|
| 0.89 | 1.96 | Sn is more electronegative than Ba on Pauling scale as, In Sn, there are two electrons in the valence p-orbital so it will attain half-filled stability after the gain of one electron so its electronegativity is higher. |
Barium and oxygen electronegativity
Oxygen is a non-metal, so a difference in the electronegativity values between Ba and O is observed.
| Electronegativity of barium | Electronegativity of oxygen | reason |
|---|---|---|
| 0.89 | 3.44 | Oxygen is the second most electronegative element in the periodic table. This is because O is a chalcogen element and is the first element of group VI A which has a high tendency to attract bonded electrons to attain a stable configuration. |
Barium and calcium electronegativity
There is only a little difference between the electronegativity of Ba and Ca as they are of same group.
| Electronegativity of barium | Electronegativity of calcium | reason |
|---|---|---|
| 0.89 | 1 | Ba and Ca both belongs to the second group (alkaline earth metals). Ca has a slightly higher electronegativity than Ba as Ca comes before in the group and as we move down the group, the metallic character of elements increases, and electronegativity decreases. |
barium ionization energy
Barium generally shows 1st and 2nd ionization energies. However, 3rd ionization energy has also been reported.
| Ionization | Ionization energy |
|---|---|
| 1st | 503 KJ/mol |
| 2nd | 965 KJ/mol |
| 3rd | 3600 KJ/mol |
The 3rd ionization energy is significantly higher as after the loss of two electrons, Ba will attain a noble gas configuration, and thus it is quite difficult to remove an electron from a stable state.
barium ionization energy graph
On plotting I.E. values against the order of ionization we will observe a sudden rise in value for 3rd ionization due to the removal of electron from a noble gas configuration.
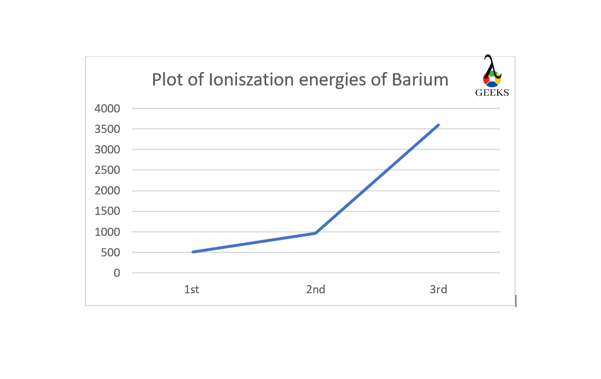
barium and beryllium ionization energy
Ba and Be both are alkaline earth metals with two electrons in valance s-orbital. The corresponding ionization energies are higher for Be as beryllium comes before barium in the group and moving down the group the Ionization energy decreases due to an increase in the number of shells.
| Ionization | Ionization energy of Ba | ionization energy of Be | reasons |
|---|---|---|---|
| 1st | 503 KJ/mol | 899.5 KJ/mol | In Be and Ba both, 1st electron is removed from the s-orbital. |
| 2nd | 965 KJ/mol | 1457.1 KJ/mol | For Be and Ba, the second electron is removed from the s-orbital giving a noble gas configuration. |
| 3rd | 3600 KJ/mol | 14847.8 KJ/mol | The 3rd ionization energy is significantly higher for both as it requires very high energy to remove an electron from any element which has attained a stable configuration. |
barium and nitrogen ionization energy
Nitrogen is the element of group 15 , so its I.E. values will be different from that of Ba
| Ionization | ionization energy for Ba | ionization energy for nitrogen | reasons |
|---|---|---|---|
| 1st | 503 | 1402.3 | 1st electron is removed from the p-orbital in the case of nitrogen. The energy is significantly higher due to half-filled stability in p-orbitals. |
| 2nd | 965 | 2856 | In N, 2nd electron is removed from p-orbitals only. |
| 3rd | 3600 | 4578.1 | The last electron is removed from the p-subshell making it empty. |
barium and radium ionization energy
Barium and radium belongs to the same group and Ra comes first so their ionization energy will differ to some extent.
| Ionization | ionization energy for Ba | ionization energy for radium | reasons |
|---|---|---|---|
| 1st | 503 | 509.3 | Only a slight difference is observed in the values as they are consecutive to each other in the group. |
| 2nd | 965 | 979.0 | The second electron removal provides a stable state in both. |
| 3rd | 3600 | Third ionization energy has not been reported for Ra. |
barium and calcium ionization energy
Barium and calcium are the elements of 2nd group. The valence electrons are present in the s-orbital in Ca and Ba. Since Ca comes first in the group, the value for the ionization energies will be comparatively higher.
| Ionization | ionization energies for Ba | ionization energies for Ca | reasons |
|---|---|---|---|
| 1st | 503 | 589.8 | The first electron is removed from the s-orbital in both cases. |
| 2nd | 965 | 1145.4 | After the removal of the second electron, they attain stability. |
| 3rd | 3600 | 4912.4 | high energy is required to remove further electrons due to the stable configuration. |
Conclusion
Barium is less electronegative than most non-metals and some metals too. The chemical properties of Ba are similar to that of Mg, Ca, and Sr. Barium is very reactive, has seven stable isotopes, and is used in removing unwanted gases from TV tubes.
Hi! I am Lubna Khan. I have done my Postgraduation in Chemistry at Jamia Millia Islamia, New Delhi. I have been in academia for years and have always welcomed new opportunities, lifestyles, and cultures coming my way.
Let us connect more at LinkedIn: