Barium, a chemical element, occurs mainly as Barytes and Witherite. Barium originates from the Greek word ‘Barys’, meaning heavy. It is a s-block element and an alkaline earth metal.
Barium electron configuration is [Xe]6s2 . It is a soft, lustrous metal. It never exists in the elemental state due to its high reactivity. It reacts vigorously with water, forms oxides reacting with air. Its melting point is approximately 727°C. Ba forms a body-centered cube. It generally forms divalent cation.
In this article, we will discuss the electronic arrangement of Barium, including ground state configuration, orbital diagram, etc. Let’s explore!
How to write Barium electron configuration
Ba is in the sixth period, and group IIA is in the periodic table with atomic number 56. According to the atom neutrality rule, in neutral Ba total of 56 electrons are present. There are some steps to follow to write the electron configuration of Ba-
Step 1- Write electronic shells
- First, we assign the electronic shells based on the principal quantum number (n). The maximum capacity of electrons in each shell follows the 2n2 rule. (n=1, 2, 3, …).
Step 2- Write electronic subshells
- Then we assign the subshells under shells. Electrons are distributed in a subshell based on the azimuthal quantum number (l). l=0, l=1, l=2 are assigned as ‘s’ subshell, ‘p’ subshell, and ‘d’ subshell, respectively. The number of subshells depends on the ‘n.’ For n=3, l values are 0, 1, 2, 3. The maximum electron capacity of an electron subshell is 2(2l+1).
Step 3- Fill electrons in orbitals
- The lowest energy orbital will be filled first, and subsequently, the others following the Aufbau principle . Hund’s rule and Pauli’s exclusion principle are imposed to take care of the maximum spin multiplicity and electron spin direction subsequently.
Barium electron configuration diagram
The electron configuration diagram of Ba consists of electrons arranged in ascending order of the energy levels. It has a total of 56 electrons. Thus,
- 1s orbital is filled out with a maximum of two electrons with opposite spins as it is the lowest energy orbital.
- After 1s orbital, 2s orbital is filled out with a maximum of two electrons with mutually opposite spins.
- After 2s orbital, 2p orbital is filled out with a maximum of 6 electrons with a total spin multiplicity equal to 1.
- After 2p orbital, 3s orbital is filled out with a maximum of 2 electrons with mutually opposite spins.
- After 3s orbital, 3p orbital is filled out with a maximum of 6 electrons with total spin multiplicity equal to 1.
- After 3p orbital, 4s orbital is filled out with a maximum of 2 electrons with mutually opposite spins.
- After 4s orbital, 3d orbital is filled out with a maximum of 10 electrons with total spin multiplicity equal to 1.
- After 3d orbital, 4p orbital is filled out with a maximum of 6 electrons with a total spin multiplicity equal to 1.
- After 4p orbital, 5s orbital is filled out with a maximum of 2 electrons with mutually opposite spins.
- After the 5s orbital, the 4d orbital is filled out with a maximum of 10 electrons with a total spin multiplicity equal to 1.
- After 4d orbital, 5p orbital is filled out with a maximum of 6 electrons with a total spin multiplicity equal to 1.
- After 5p orbital, 6s orbital is filled out with a maximum of 2 electrons with mutually opposite spins.

Barium electron configuration notation
The electronic configuration notation of Ba is [Xe]6s2.
The electron configuration of Ba includes a total of 56 electrons. Fifty-four electrons come from Xenon noble gas atom, and two from the 6s orbital. It is also known as abbreviated electron configuration.
Barium unabbreviated electron configuration
The unabbreviated electron configuration of Ba is,
1s22s22p63s23p64s23d104p65s24d105p66s2.
The unabbreviated electron configuration of Ba are filled as-
- Two electrons in 1s orbital.
- Two electrons in 2s orbital.
- Six electrons in 2p orbital
- Two electrons in 3s orbital
- Six electrons in 3p orbital
- Two electrons in 4s orbital
- Ten electrons in 3d orbital
- Six electrons in 4p orbital
- Two electrons in 5s orbital
- Ten electrons in 4d orbitals
- Six electrons in 5p orbital
- Two electrons in 6s orbital
Ground state Barium electron configuration
- The ground state electron configuration of Ba is
1s22s22p63s23p64s23d104p65s24d105p66s2
- In terms of electron shell, electron configuration of Ba in ground state is:
K- level = 2, L-level = 8, M-level = 18, N-level = 18, O-level = 8, P-level = 2
Excited state Barium electron configuration
The excited electron of Ba occupies the 5d orbital, and the electronic configuration becomes 1s22s22p63s23p64s23d104p65s24d105p66s15d1.

Ground state Barium orbital diagram
Ground state electronic shell structure of Ba is 2.8.18.18.8.2. The ground state term symbol for Ba is 1S0. The shell diagram is shown below,

Barium chloride electron configuration
- The electronic configuration of Ba2+ is: 1s22s22p63s23p64s23d104p65s24d105p6
- The electronic configuration of Cl– is: 1s22s22p63s23p6
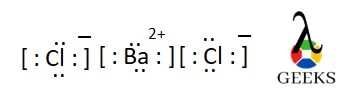
Conclusion
We can write the electron configuration of Ba in terms of the electron shell model, noble gas notation, unabbreviated form, etc. Ba readily loses two electrons to attain noble gas configuration. It is used in paint and glassmaking.
Also Read:
- Oxygen electron configuration
- Yttrium electron configuration
- Mercury electron configuration
- Radon electron configuration
- Cadmium electron configuration
- Bromine electron configuration
- Cesium electron configuration
- Astatine electron configuration
- Gd3 electron configuration
- Curium electron configuration

Hey readers, I am Ishita Ghosh. I have done my Master’s in Chemistry. My area of specialization is Inorganic Chemistry. The true way to comprehend chemistry is to understand it from its grassroots level. My effort is to share every bit of knowledge in chemistry I have so that it helps you for a better grasp on this subject.