This article would present a set of simple diffusion examples which would help in the better understanding of types of diffusions in a detailed manner.
Diffusion is described as the process where the movement of the particles are from the higher concentration level to a lower concentration level. The differences in the concentration of the two areas are termed as the concentration gradient and the process of diffusion continues till the neutralisation of the gradient.
Passive transport and types
Passive transport is explained as the movement of the ions or a set of molecular substances within the cells along a specific concentration gradient without the requirement of any form of eternal energy. There are four types of passive transport, simple diffusion, facilitated diffusion, filtration and osmosis.
Simple diffusion
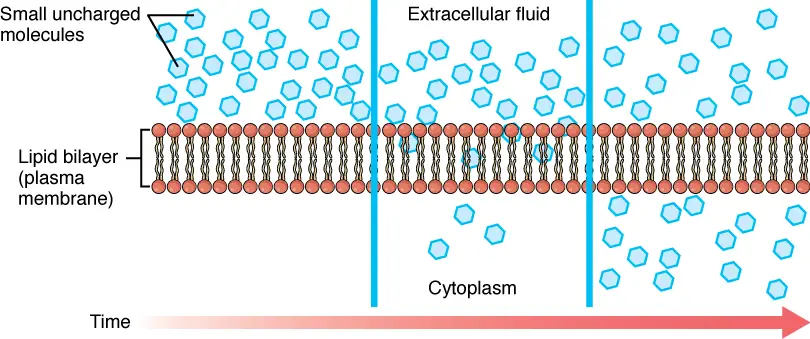
Simple diffusion is the basic movement of the ions or molecular particles from a higher concentration region to a lower concentration region. In cells, the movement is across a semipermeable membrane and occurs without the support of any protein channels.
For example, diffusion tends to occur in both liquid and gases as the particles have the ability to move freely from one place to the other in a random manner. Similarly, in cells, the movement of the particles occurs in and out through simple diffusion.
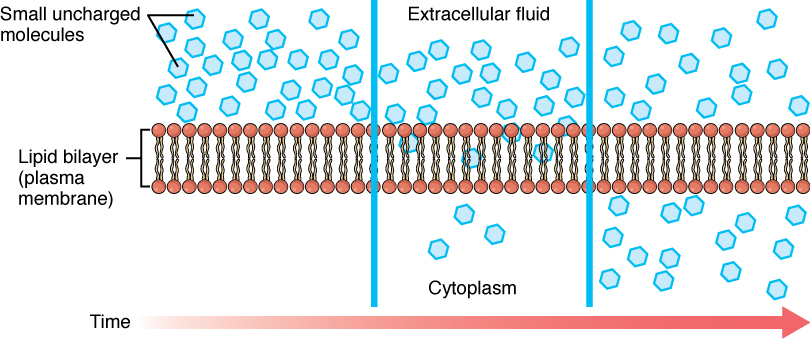
Facilitated Diffusion
Facilitated diffusion is the type where the transportation of the ions across the semipermeable membrane is done with the support of specific sets of transmembrane integral proteins. These molecules are mostly large in size and are dependent on the carrier substance to pass through the membrane.
There is no requirement of any external energy for this process. For example, glucose transport or amino acid transport are carried with the help of facilitated diffusion. The cell membrane is permeable to molecules that are non-polar and smaller in size.
Filtration
The selective absorption of few sets of nutrients within the body is termed as filtration. This process has no requirement of external energy and occurs through the concentration gradient. Here, mostly the solids are filtered from liquids and gases. The substances are mostly soluble in nature and hence can pass easily through the pores.
For example, in our body, the kidney establishes filtration. Here, the blood gets filtered by the glomerulus where the necessary molecules for the body are reabsorbed.
Osmosis
Osmosis is the process where the water molecules along with other kinds of molecules pass through the semipermeable membrane from a higher concentration to a lower concentration in order to balance the overall contraction level. Osmosis is directly proportional to the concentration gradient and temperature. The greater is the concentration gradient and temperature, the faster would be the process of osmosis.
For example, there is the absorption of water from the soil by the plants due to the process of osmosis. The concentration of the water is lower in the roots than the soil, hence the flow of water is made into the roots which is further utilised by the plants.
Simple diffusion examples
Few of the simple diffusion examples in our body are as follows:
Oxygen and Carbon dioxide
It has been identified that movement of gases across the membranes are among the most classic examples for simple diffusion within animals. Both the oxygen and carbon dioxide get dissolved in the blood in order to process the exchange of gases using the simple diffusion method.
The direction regarding the movement of gases is dependent on the concentration gradient of the gases within the cells. During the time of inhalation, the concentration of oxygen is greater than carbon dioxide within the alveoli than the blood vessels. Hence, the movement of oxygen is into the blood from the alveoli.
During the time of exhalation, the concentration of the carbon dioxide is greater than oxygen within the blood vessels than the alveoli. Hence, the movement of oxygen is into the alveoli of the lungs from the blood, which is then exhaled out through windpipe.
Movement of waste materials
The process of removing waste from the body within animals is processed using simple diffusion. In the liver, the set of waste material called urea is excreted from the blood. In a similar manner, the waste like toxins and chemicals are removed from the kidneys and the absorption of water is processed by the method of simple diffusion. It is often found in collaboration with active transport and osmosis as well.
Bacteria
Bacteria are simple organisms which leave them with a limited number of ways to intake the necessary nutrients. Hence, the nutrients are diffused across the cell membrane. Bacteria lack the specialised organelles in transporting substances and hence the reliance is more on simple diffusion.
Bacteria tend to include facilitated diffusion as well in transporting most nutrients but simple diffusion is important in terms of delivering water, oxygen and few of the small sized nutrients within the cytoplasm.
Conclusion
The article concludes that simple diffusion forms an important process in terms of managing life forms on earth which can be noticed through various simple diffusion examples.
Also Read: