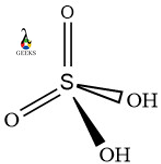
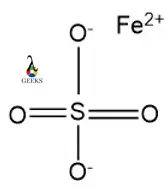
15 Facts on H2SO4 + Cu: What, How To Balance & FAQs
In a reaction sequence, one or more products are generally formed. Let us see how the reaction of H2SO4 and Cu occurs. Sulphuric acid mainly exists as an aqueous solution as it is hygroscopic in nature. Copper(Cu) is a transition metal with a reddish lustre,playing a huge role in industries. Copper does not undergo corrosion … Read more