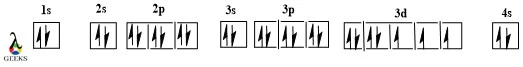
Constitutional isomers examples have different connectivity and can be defined as those molecules which have the same molecular formula but different structural representations or bonding arrangements. Based on the arrangement they are bifurcated into 3 types skeletal isomers, positional isomers, and functional isomers.
- Butane and isobutane constitutional isomers examples
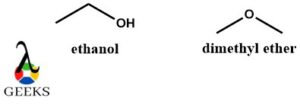
- Ethanol and dimethyl ether constitutional isomers examples
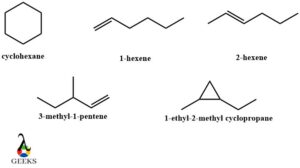
- C6H12 constitutional isomers examples
- N-pentane, isopentane, neo-pentane constitutional isomer examples
- Propionic acid and 1-hydroxy-2-propanone constitutional isomers examples
- C5H10O constitutional isomers examples
- C4H8 constitutional isomers examples
- C6H14 constitutional isomers examples
- 1-butene and 2-butene constitutional isomers examples
- N-methyl cyclohexanone constitutional isomers examples
- Glucose and fructose constitutional isomers examples
- Nitromethane and methyl nitrite constitutional isomers examples
Butane and isobutane constitutional isomers examples
Butane and isobutane are constitutional isomers examples and consist of only carbon and hydrogen in their structure. Their molecular formula is the same i.e C4H10. They differ in the length of the chain and the position of the methyl group. Hence isobutane according to the IUPAC nomenclature is designated as 2-methyl propane. Isobutane is branched and butane is a linear chain compound.

Ethanol and dimethyl ether constitutional isomers examples
Ethanol and dimethyl ether are suitable constitutional isomers examples. They have the same molecular formula C2H6O but completely different structures, physical properties, and chemical properties. Along with being constitutional isomers examples, they are functional isomers as they belong to 2 different functional groups one with the -OH group and the other with the ether group.
C6H12 constitutional isomers examples
C6H12 constitutional isomers examples can form many aliphatic and cyclic compounds with the organization of atoms and bonds. The constitutional isomers example is Cyclohexane, 1-hexene, 2-hexene, 3-methyl-1-pentene, and 1-ethyl-2-methyl cyclopropane. These constitutional isomers examples can be best explained diagrammatically.
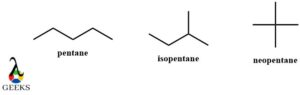
N-pentane, isopentane, neo-pentane constitutional isomer examples
These are the most common constitutional isomers examples. Here the n-pentane is the normal linear molecule and isopentane is the branched molecule with the shifting of a single methyl group. The neopentane is a criss-cross structure molecule that is highly branched and has a central carbon atom surrounded by 4 CH3 groups. The entire constitutional isomers examples have the same molecular formula.
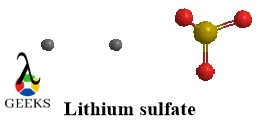
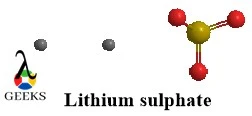
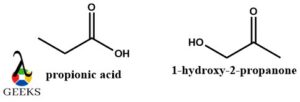
Propionic acid and 1-hydroxy-2-propanone constitutional isomers examples
Both of these constitutional isomers examples belong to 2 different functional groups one being carboxylic acid and the other being ketone. They not only show structural variation but a difference in their properties as well despite having the same molecular formula.
C5H10O constitutional isomers examples
C5H10O constitutional isomers examples usually belong to ketones and aldehydes functional groups. There are a total of 7 isomer structures out of which 4 are aldehydes and 3 are ketones.

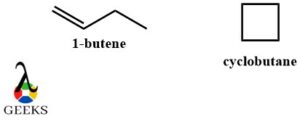
C4H8 constitutional isomers examples
C4H8 molecular formulas usually have 2 most popular constitutional isomers examples. One of the structures is an alkene with a double bond and the other one is a cyclic compound. Their names are butene and cyclobutane respectively.
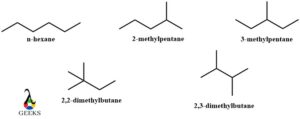
C6H14 constitutional isomers examples
C6H14 is an alkane named hexane. It has 5 constitutional isomers examples namely n-hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, 2,3-dimethylbutane. They are best described diagrammatically.
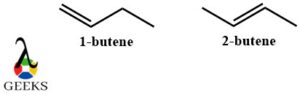
1-butene and 2-butene constitutional isomers examples
In the above constitutional isomers examples, the molecular formula C4H8 is the same. The only difference is the position of the double bond. In 1-butene it is at the end of the structure and in 2-butene it is in the middle. So it also shows positional isomerism.
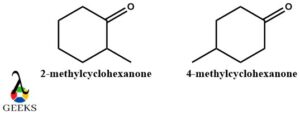
N-methyl cyclohexanone constitutional isomers examples
Here 2 constitutional isomers examples are observed where there is a difference in the position of functional group CH3 and OH concerning each other. They form ortho and para organic compounds 2-methylcyclohexane and 4-methylcyclohexane respectively.
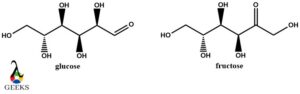
Glucose and fructose constitutional isomers examples
Glucose and fructose are both carbohydrates with the molecular formula C6H12O6. They are very suitable constitutional isomers examples as they only differ in the position of the double bond oxygen atom. So they can be called regioisomers due to positional differences.
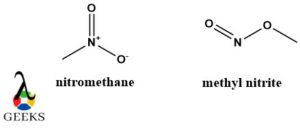
Nitromethane and methyl nitrite constitutional isomers examples
Both of these constitutional isomers examples have the same molecular formula CH3NO2 and functional group NO2 but the atoms are connected in different order and arrangements.
Conclusion
So, in nutshell constitutional isomers examples are structural isomers where the molecular formula is same but there is difference in structural representation and the arrangement of atoms and the bonds in the organic compounds. These arrangements can vary according to the change in functional groups (Functional isomers), no. of chains (Chains isomers) and the position of the groups (regioisomers).