5 Facts On Beryllium Ionization Energy & Electronegativity
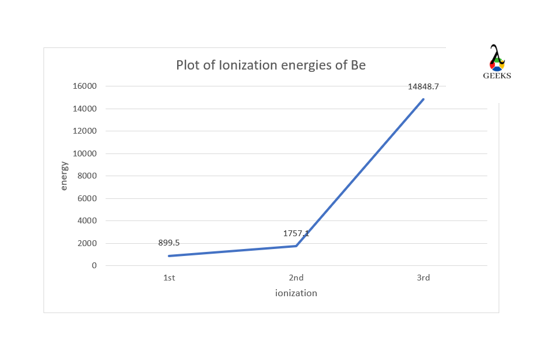
Beryllium (Be) is the first element of the second group in the periodic table and is a soft metal. Let us study some facts about Be in detail. Be generally shows 1st and 2nd ionizations, these ionization energies are 899.5 KJ/mol and 1757.1 KJ/mol, respectively, where the electrons are removed from the valence s-orbital for … Read more