In this article we are going to see about alcl4- lewis structure and some important facts around this.
Lewis structure or lewis dot structure is a simple representation of the electronic structure of a molecule that briefs about the number of bonds formed, number of bond pairs required to fulfill the octet rule and lone pairs available. This method of drawing a molecule helps in simple representation of a molecule by allowing to guess its structure or shape.
An atom always tend to arrange 8 electrons around themselves to acquire a stable or a noble gas configuration with some exceptions like when a molecule is electron deficient; when it has odd number of electrons; or molecules that has extra electrons in their valence shells. E.g., BH3 ,SF6 ,NO etc.
Methods to draw a Lewis structure :
- First count the total number of valence shell electrons available for each atom.
- Choose the least electronegative atom as the central atom and draw the remaining atoms around the central atom and start by forming a covalent bond (a bond requires two electrons). An atom will always try to fulfill its octet or expand its octet if required.
- The remaining electrons not forming covalent bond will stay as lone pair of electrons.
Note: Elements having expanded valence shells like 3d elements, it can exceed the octet rule like SF6 , PF5 or elements with fewer valence electrons can have incomplete octet like H2 .

Lewis structure of AlCl4– :
Aluminium belongs to 13th group and 3rd period. It has 3 valence electrons, with an empty 3d shell that can expand its octet if required ( but it does not due to steric hindrance.) It has 4 Cl atoms with electronegativity 3.16 and Al with electronegativity 1.61, therefore, choose Al as the central atom. It has a total of 32 valence electrons from 4 Cl atoms, 1 Al atom and a negative charge. Draw a covalent bond between each atoms with the central atom to fulfill the octet. In doing so, we get 12 lone pairs of electrons, 3 each on the surrounding atoms. Each atom’s octet is filled thereby not violating the octet rule.
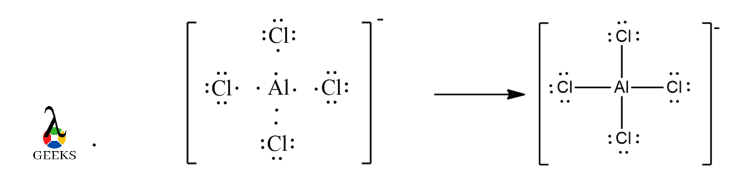
Formal Charge of AlCl4– :
It briefs about the hypothetical charge acquired by an atom in a molecule if the electron pairs were shared evenly between the atoms to fill its valency completely.
Generally, formal charge can be calculated mathematically by the formula :
Formal charge = (Number of valence electrons in a free atom of the element) – (Number of unshared electrons on the atom) – (Number of bonds to the atom)
In addition, Charge on the molecule= sum of all the formal charges .
Formal charge of Al atom = 3-0-4 = -1
Formal charge of Cl atom = 7-6-1= 0
Since all the chlorine atoms are equivalent, hence we assign 0 formal charge to all the Cl atoms ( meaning they are neutral )
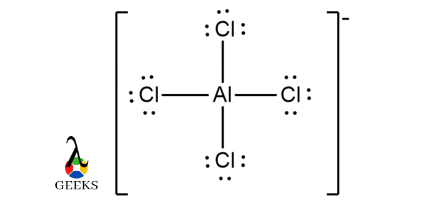
Resonance structure of AlCl4– :
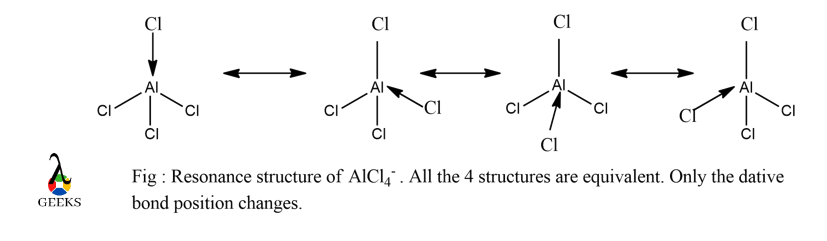
Aluminium has valence shell configuration 3s2 3p1 . It generally shows a covalency of 3 acting as a Lewis acid but it can accommodate extra electrons in its empty 3d orbital and two 3p subshells thereby extending its covalency to 8. However, Al has small size which cause steric hindrance with greater covalency.
Therefore, it sticks to its minimum covalency of 3 and if an extra electron is provided by a donor, it can extend its covalency upto 6. In our case of Tetrachloroaluminate, it extends its covalency to 4. Therefore, it doesn’t form any double or triple bonds due to steric hindrance. All the 4 resonance structures are equivalent which is shown below :
This form is the most stable orientation of AlCl4– . The `arrow’ is the coordinate/dative bond.
Note: Dative or coordinate bonds are formed by sharing two electrons covalently by an atom to the nearest neighbouring atom.
AlCl4– valence electrons, bond pairs and lone pairs of electrons :
It has a total of 32 valence electrons.
It has 4 bond pairs ( that is formed by a single covalent bond here) and 12 total lone pairs of electrons ( that did not participate in bond formation).
Note: In actual sense of chemistry, there is a coordinate bond formation between AlCl3 and Cl– ( electron pairs shared completely by chloride ion, a type of covalent bond).
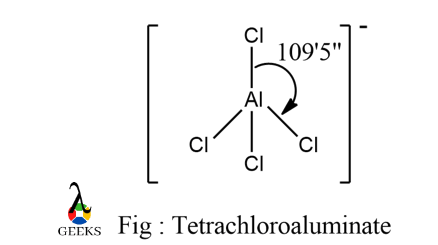
Hybridisation, Shape and Angle of AlCl4– :
There is a simple rule or equation to be followed to find out the hybridization of molecules real quick.
Hybridisation of a molecule = ( Valence electrons of the central atom + Number of monovalent atoms attached to the central atom + Negative charge on the molecule – Positive charge on the molecule )/2
Here, AlCl4– hybridization = (3+4+1)/2 = 4 i.e., sp3
It is a tetrahedral structure and not square planar as alkaline earth metals do not necessarily exhibit square planar complexes formation since they do not contribute to the overall CFSE value.
It has sp3 hybridisation with tetrahedral geometry and bond angle of 109’5” . It has 4 single bonds with bond length of Al-Cl at around 2.06-2.08 armstrong.
| Molecule | Tetrachloroaluminate, AlCl4– |
| Type | Polyatomic ion |
| Hybridisation | sp3 |
| Shape | Tetrahedral |
| Bond angle | 109’5” |
| Bond and Lone Pairs | 4 , 12 |
Is AlCl4– stable ?
Yes, AlCl4– ion is found to be stable. It has high thermal stability, high conductivity, low melting point which make it an excellent choice for further study and investigation. It has its octet complete making it a stable compound.
Note : Formation of AlCl4– – As AlCl3 has empty p orbital that can accept another electron to complete its octet, it reacts with a chloride ion easily to form a more stable ionic molecule. They are also observed as an intermediate species during many organic reactions like Friedel Crafts alkylation, acylation etc.
Is AlCl4– ionic or covalent ?
Tetrachloroaluminate is a covalent molecule. It is a polyatomic anion where a chloride ion shares two electrons by a covalently coordinate bond to AlCl3(covalent) .
Note: A polyatomic anion with a metal cation yields an ionic compound.
Uses of AlCl4– :
- Due to its thermal stability, low melting point and low vapor pressure, it is widely used as an ionic electrolyte for redox reactions and chemical reactions.
- It is used in solvent extraction, organic catalysis reaction, in Al dual-ion batteries.
- They are used in batteries
Conclusion :
AlCl4– or Tetrachloroaluminate is a sp3 hybridised polyatomic molecule with tetrahedral geometry containing 4 bond pairs and 12 lone pairs which is widely used for commercial and industrial purposes.
Also Read:
- Ch2f2 lewis structure
- Brf4 lewis structure 2
- Ch2i2 lewis structure
- Scn lewis structure
- Al2s3 lewis structure
- Cli3 lewis structure
- Lih lewis structure
- Clo4 lewis structure
- Carbonic acid lewis structure
- Xeo3 lewis structure 2

Hello…. I am Nandita Biswas. I have completed my master’s in Chemistry with a specialization in organic and physical chemistry. Also, I have done two projects in chemistry- One dealing with colorimetric estimation and determination of ions in solutions. Others in Solvatochromism study fluorophores and their uses in the field of chemistry alongside their stacking properties on emission. I am working as a Research Associate Trainee in Medicinal Department.
Let’s connect through LinkedIn-https://www.linkedin.com/in/nandita-biswas-244b4b179