Topic of Discussion: Adiabatic Process
- Adiabatic process definition
- Adiabatic process examples
- Adiabatic process formula
- Adiabatic process derivation
- Adiabatic process work done
- Reversible adiabatic process and Irreversible adiabatic process
- Adiabatic graph
Adiabatic process definition
Abiding the first law of thermodynamics, the process occurring during expansion or compression where there is no heat exchanged from the system to the surroundings can be known as an adiabatic process. Differing from the isothermal process, adiabatic process transfers energy to the surrounding in the form of work. It can be either reversible or an irreversible process.
In reality, a perfectly adiabatic process can never be obtained since no physical process can happen spontaneously nor a system can be perfectly insulated.
Following the first law of thermodynamics that says when energy (as work, heat, or matter) passes into or out of a system, the system’s internal energy changes accordingly with the law of conservation of energy, where E can be denoted as the internal energy, while Q is the heat added to the system and W is the work done.
ΔE=Q−W
For an adiabatic process where there is no heat exchanged,
ΔE=−W
Conditions required for an adiabatic process to take place are:
- The system must be completely insulated from its surroundings.
- For the heat transfer to occur in a sufficient amount of time, the process must be performed quickly.
Adiabatic process Example
- Expansion process in an internal combustion engine found among hot gases.
- The quantum-mechanic analogue of an oscillator classically known as the quantum harmonic oscillator.
- Gases liquified in a cooling system.
- Air released from a pneumatic tire is the most significant and common instance of an adiabatic process.
- Ice stored in an icebox follows the principles of heat not being transferred in and out to the surroundings.
- Turbines, using heat as a medium to generate work, is considered an excellent example as it reduces the efficiency of the system as the heat is lost to the surroundings.
Adiabatic process formula
The expression of an adiabatic process in mathematical terms can be given by:
ΔQ=0
Q=0,
ΔU= -W, (since there is no heat flow in the system)
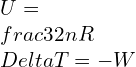
Therefore,
![]()
Consider a system where the exclusion of heat and work interactions on a stationary adiabatic process is performed. The only energy interactions are the boundary work by the system in its surroundings.
![]()
![]()
Ideal gas
The amount of thermal energy per unit temperature unavailable to perform specific work can be defined as the entropy of a system. A speculative gas that comprises the random motion of point particles subject to interparticle molecular interactions is ideal.
The molar form of the ideal gas formula is given by:
![]()
![]()
![]()
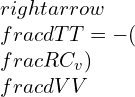
Integrating the equations,
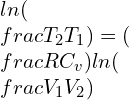
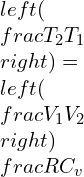
Adiabatic process equation can be denoted as:
PVY = constant
Where,
- P= pressure
- V= volume
- Y= adiabatic index; (Cp/Cv)
For a reversible adiabatic process,
- P1-YTY = constant,
- VTf/2 = constant,
- TVY-1 = constant. (T = absolute temperature)
This process is also known as the isentropic process, an idealized thermodynamic process containing frictionless work transfers and adiabatic. In this reversible process, there is no transfer of heat or work.
Adiabatic process derivation
The alteration in internal energy dU in a system to work done dW plus the heat added dQ to it can be associated as the first law of thermodynamics through which the adiabatic process can be derived.
![]()
According to the definition,
![]()
Hence,
![]()
Addition of heat escalates the amount of energy U defining the specific heat as the amount of heat added for a unit rise in temperature change for 1 mole of a substance.
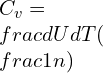
(n is the number moles), Therefore:
![]()
Derived from the ideal gas law,
![]()
![]()
Merging equation 1 and 2,
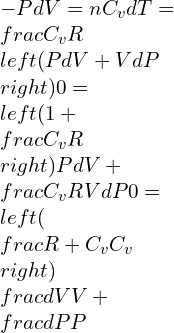
For a constant pressure Cp, heat is added and,

γ is the specific heat
![]()
Using the integration and differentiation concepts, it is arrived at:
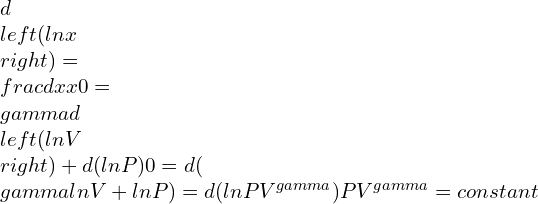
This equation above becomes real for a given ideal gas that contains the adiabatic process.
Adiabatic process Work done.
For a pressure P and a cross-sectional area A moving through a small distance dx, the force acting would be given by:
![]()
And the work done on the system can be written as:
![]()
Since,
![]()
The net work produced for the expansion of the gas from the volume of the gas Vi to Vf (initial to the final) will be given as
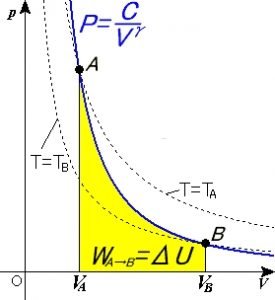
W= area of ABDC from the graph plotted as the adiabatic process takes place. The conditions to be followed are associated with an example of a perfectly non-conducting piston cylinder with a single gram molecule of a perfect gas. The cylinder’s container is to be made of an insulating material, and the curve plotted by the graph should be sharper.
Whereas, in an analytical method to derive the work done on the system would be as follows:
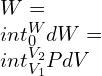 —–(1)
—–(1)
Initially, for an adiabatic change, we can assume:
![]()
Which can be,
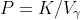
From (1),
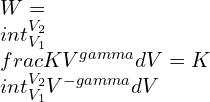
![Rendered by QuickLaTeX.com W=kleft | frac{V^{1-gamma }}{1-gamma } right |=frac{K}{1-gamma }left [ V_{2}^{1-gamma }-V_{1}^{1-gamma } right ]](https://lambdageeks.com/wp-content/ql-cache/quicklatex.com-4b36353a4188600233e8d19d1e3e334c_l3.png)
For solving,
![]()
Thus,
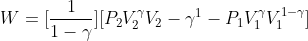
Which is,
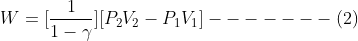
Taking T1 and T2 as the initial and final temperatures of the gas respectively,
![]()
Using this in equation (2),
![Rendered by QuickLaTeX.com W=left [ frac{R}{1-gamma } right ]left [ T_{2}-T_{1} right ]](https://lambdageeks.com/wp-content/ql-cache/quicklatex.com-8679105960c7b3c775fdb83e49bec52d_l3.png)
Or,
![Rendered by QuickLaTeX.com W=left [ frac{R}{gamma-1 } right ]left [ T_{1}-T_{2} right ]](https://lambdageeks.com/wp-content/ql-cache/quicklatex.com-4fcbba1eadf9f6863cc1a7ace54364b0_l3.png) —-(3)
—-(3)
The heat required during the expansion process to do the work is:
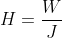
![Rendered by QuickLaTeX.com =left [ frac{R}{J(gamma-1)} right ]left [ T_{1}-T_{2} right ]](https://lambdageeks.com/wp-content/ql-cache/quicklatex.com-d976bfeaa8810ac3147fe3e25e46a4e5_l3.png)
As R is the universal gas constant and during adiabatic expansion, the work done is directly proportional to the decrease in temperature, while the work done during an adiabatic compression is negative.
Hence,
![Rendered by QuickLaTeX.com W=-left [ frac{R}{gamma-1} right ]left [ T_{1}-T_{2} right ]](https://lambdageeks.com/wp-content/ql-cache/quicklatex.com-cd06dd3f1e3f719968f81ce3d0fb491f_l3.png)
Or,
![Rendered by QuickLaTeX.com W=-left [ frac{R}{1-gamma} right ]left [ T_{2}-T_{1} right ] ----left ( 4 right )](https://lambdageeks.com/wp-content/ql-cache/quicklatex.com-b3078db835607f577fa0f7352f57139d_l3.png)
This can be given as the work done in an adiabatic process.
And the heat expelled during the process is:
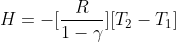
Adiabatic graph
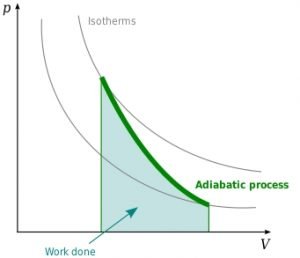
image credit : User:Stannered, Adiabatic, CC BY-SA 3.0
The mathematical representation of the adiabatic expansion curve is represented by:
![]()
P,V,T are the pressure, volume, and temperature of the process. Considering the initial stage conditions of the system as P1, V1, and T1, also defining the final stage as P2, V2, and T2 respectively the P-V graph diagram is plotted essentially for a piston cylinder movement heated adiabatically from the initial to final state for a m kg of air.
Adiabatic entropy, adiabatic compression and expansion
A gas allowed to expand freely without the transfer of external energy to it from higher pressure to a lower pressure will essentially cool by the law of adiabatic expansion and compression. Likewise, a gas will heat up if it is compressed from a lower temperature to a more significant temperature without the substance’s transfer of energy.
- Air parcel will expand if the surrounding air pressure is reduced.
- There is a decrease in temperature at higher altitudes due to the diminish in the pressure as they are directly proportional in the case of this process.
- Energy can either be utilized to do work for expansion or to maintain the temperature of the process and not both at the same time.
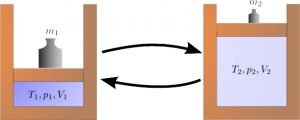
Reversible adiabatic process
![]()
The frictionless process where the system’s entropy remains constant is coined as the term reversible or isentropic process. This means that the change in entropy is constant. The internal energy is equivalent to the work done in the expansion process.
Since there is no heat transfer,
![]()
Thus,
![]()
Which means that,
![]()
Examples of a reversible isentropic process can be found in gas turbines.
Irreversible adiabatic process
As the name suggests, the internal friction dissipation process resulting in the change in entropy of the system during the expansion of gases is an irreversible adiabatic process.
This generally means the entropy increases as the process furthers that cannot be performed at equilibrium and cannot be tracked back to its original state.
To know about Thermodynamics click here

The lambdageeksScience Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.