Adiabatic compression and expansion are two processes famous in thermodynamics.
In this process, The substance is expanded without heat transfer. The Carnot, Diesel, Otto are examples of the adiabatic process.
The main processes of work done is adiabatic in the thermodynamics. one is a reversible adiabatic process, and another is an irreversible adiabatic expansion.
The irreversible adiabatic process occurs in the free expansion of gas.
What is adiabatic expansion?
The adiabatic process in thermodynamic is used in various cycle
It is the expansion of substance in the system with no heat or mass transfer with the surrounding.
This concept is well understood in the study of the heat engine. The adiabatic expansion is an idealized process with no heat transfer.
In actual practice, the expansion of the substance is caused in a system very speedy. This process is occurring quickly, so the exchange of the heat from the system to the surrounding is minimal. The heat flow through the boundary is significantly less. This process is considered adiabatic expansion.
Adiabatic expansion formula
There are many possible conditions for the adiabatic expansion formula.
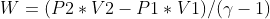
Some assumptions are made for driving the equation for the adiabatic expansion process.
The wall of the system is insulating
The wall of the system (cylinder) is frictionless
If piston travel up by distance dx due to the action of the pressure P
The work done in the system can be given as,
dW = P A dx
Here, A is the cross sectional area over the piston top,
we can write A dx = dV = Change in volume
dW = P dV
The expansion of the substance is adiabatic; the state of the substance changed from the P1, V1, T1 to P2, V2, T2.
Condition of the adiabatic process, P Vϒ = Constant = K
The total work on the system can be given as,
Use P = K * V-ϒ
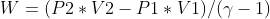
Adiabatic expansion process
This process is possible in engine, refrigeration & air conditioning
The expansion of the gas is very fast, so the exchange of heat is negligible between the system and surroundings.
There are two processes adiabatic compression and adiabatic expansion. Both processes are carried out with minimum heat transfer at the boundary in actual practice.
The free adiabatic expansion process’s fundamental is somewhat different from adiabatic expansion.
Suppose we fill gas in one box and join another empty box with it. Both boxes have the same wall. Suppose we puncture the common wall, the gas from one box start to expand in the second box. This expansion process is called free expansion.
This expansion process is caused due to volume, so pressure becomes zero. There is no work done due to the absence of pressure. If this box or system is thermally insulated, the process is known as free adiabatic expansion.
The heat transfer Q = 0, Work done W = 0
Adiabatic expansion ratio
There is two specific heat in thermodynamic processes.
The specific heat ratio at constant pressure to specific heat at constant volume is known as an adiabatic index or specific heat ratio.
If Cp = The value of specific heat at constant pressure
Cv = The value of the specific heat at constant volume
ϒ = Ratio of the two specific heat or adiabatic index
ϒ = Cp / Cv
The adiabatic index is 1.7 for the monatomic ideal gas like argon, helium.
Adiabatic expansion temperature change
The temperature of the system will affected if the system exchanges heat.
There is no exchange of heat in the this process but the work done in the expansion is due to a reduction in temperature.
The internal energy of the adiabatic expansion process is lower than the isothermal process. There is no exchange of heat with minor work done.
If the expansion process is free, the temperature remains constant. The entropy of the system has direct relation with volume if the temperature is constant. This process is irreversible due to an raise in entropy.
Adiabatic expansion work
The work done in the process is a function of heat transfer and internal energy.
In the adiabatic process, the heat transfer is zero. Work done = Change in the Internal energy.
The expansion work of the adiabatic process is given below,
Adiabatic expansion of gas
The adiabatic free expansion of the substance like gas is a straightforward concept to understand.
The gas is expanded in the vacuum without external pressure. The work is zero in this process because the external pressure is zero. W = P * dV
If filled gas from the container is allowed to expand freely in the space, there is no external pressure acting on gas.
Work done = Pressure * Change in volume
pressure = 0, so the work done on or from the system is zero.
In an adiabatic process, the transfer of heat is not possible,
According to the Ist law of thermodynamics,
ΔQ – ΔW = ΔU
Where ΔQ = Zero and ΔW = Zero
So the change in internal energy = Zero.
Adiabatic expansion of an ideal gas
The behavior of the process is changed if the gas is ideal.
The expansion of the ideal substance like ideal gas is a constant temperature process (isothermal process)
We generally consider the isentropic and the adiabatic process the same, but it is not the same in all cases. Let’s consider the example of the expansion of the ideal gas.
We consider some assumptions for this process,
- The cylinder and the piston is frictionless
- There is a vacuum outside of the piston and the cylinder
- The piston and the cylinder are thermally insulated
- There is no transfer of heat between the system and the environment (Adiabatic process)
If filled gas is allowed to expand through pushing the piston, The gas expands due to volume without any external pressure. This process is an example of increased entropy and an irreversible process.
Adiabatic irreversible expansion
In the irreversible process, the initial stage is not restored after completing the process.
The entropy of the system is varying due to friction. This process is not slow like quasi-static.
External pressure for an ideal gas is constant in the adiabatic expansion process.
The adiabatic irreversible expansion process is isothermal.
Adiabatic expansion example
Their many processes in engineering are considered adiabatic expansion.
- Air release from the tire or container
- Expansion of gas in gas turbine adiabatically
- Expansion in steam nozzle & turbine
- expansion inside piston-cylinder arrangement with an assumption
- Free adiabatic expansion of gas contained in a container
- Expansion process in heat engine with an assumption
- Adiabatic heating and cooling system
- Expansion device

I am Deepak Kumar Jani, Pursuing PhD in Mechanical- Renewable energy. I have five years of teaching and two-year research experience. My subject area of interest are thermal engineering, automobile engineering, Mechanical measurement, Engineering Drawing, Fluid mechanics etc. I have filed a patent on “Hybridization of green energy for power production”. I have published 17 research papers and two books.
I am glad to be part of Lambdageeks and would like to present some of my expertise in a simplistic way with the readers.
Apart from academics and research, I like wandering in nature, capturing nature and creating awareness about nature among people.
Also refer my You-tube Channel regarding “Invitation from Nature”