Zirconium is a crucial element in various industries, from nuclear engineering to material science, and its density is a critical property that determines its suitability for different applications. In this comprehensive guide, we will delve into the intricacies of zirconium density, exploring its measurement, factors that influence it, and its practical implications.
Understanding Zirconium Density
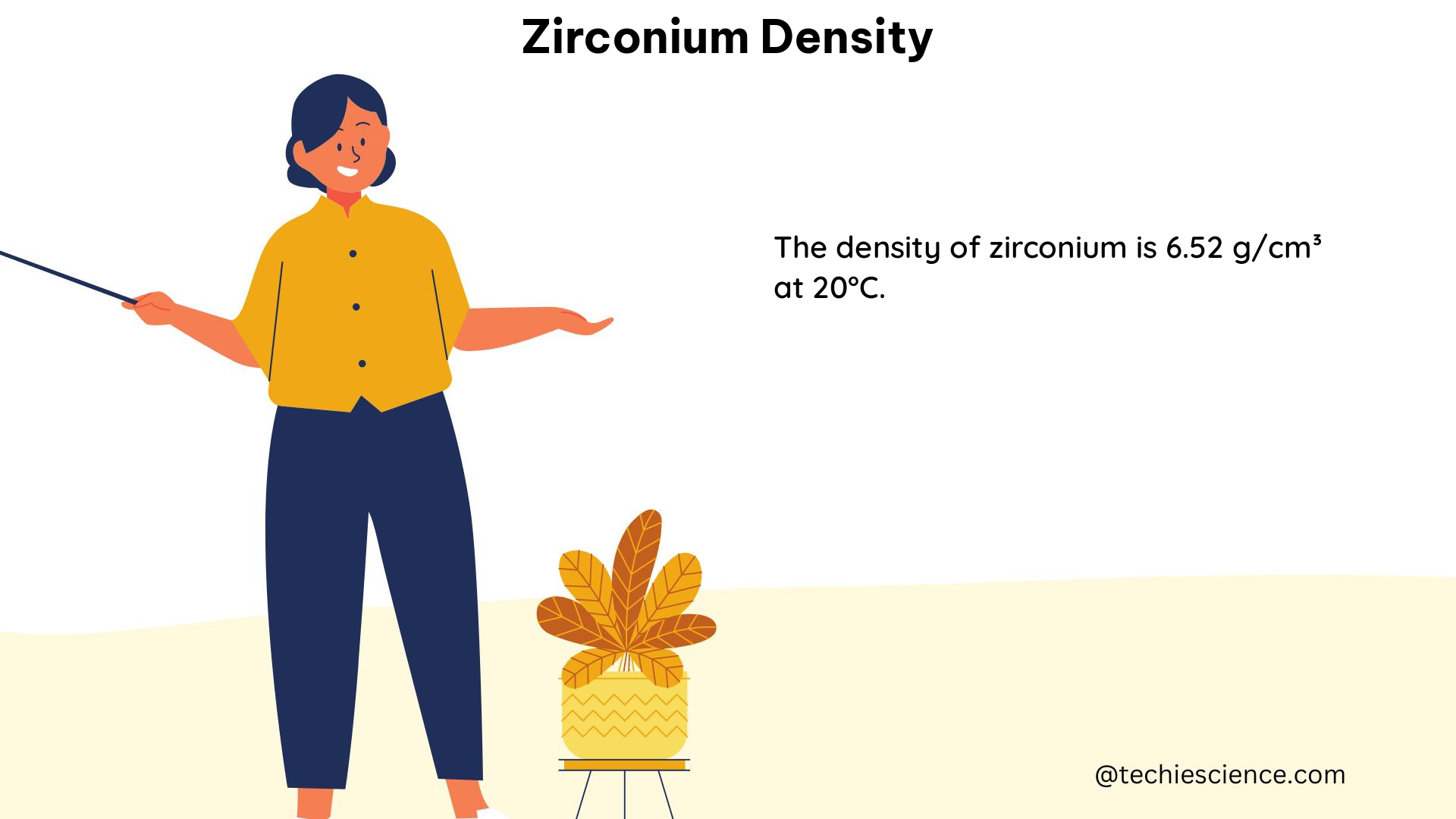
The density of zirconium is approximately 6.51 g/cm^3 at room temperature. This value is derived from careful measurements and calculations of the material’s mass and volume. However, it is essential to note that the density of zirconium can vary depending on the temperature and phase of the material.
Temperature and Phase Dependence
The density of zirconium, particularly in its liquid form, exhibits a linear relationship with temperature. The density of liquid zirconium oxide (ZrO2) can be described by the equation:
ρ = ρm – a(Tm – T)
Where:
– ρ is the density of the liquid ZrO2
– ρm is the density at the melting temperature
– Tm is the melting temperature
– T is the current temperature
– a is a free parameter
This equation demonstrates that as the temperature increases, the density of liquid zirconium oxide decreases linearly.
Atomic Weight and Crystalline Structure
In addition to temperature and phase, the density of zirconium is also influenced by its atomic weight and crystalline structure. The atomic weight of zirconium is 91.22 g/mol, which is a crucial factor in determining its density.
Zirconium exhibits two main crystalline structures: the hexagonal close-packed (HCP) structure at room temperature and the body-centered cubic (BCC) structure at higher temperatures (above 862°C). These structural changes can also affect the material’s density.
Measurable and Quantifiable Data
Alongside density, there are several other measurable and quantifiable data points related to zirconium that are essential to understand its properties and behavior.
Melting and Boiling Points
The melting point of zirconium is 1857°C, while its boiling point is 3577°C. These values are crucial in determining the material’s suitability for high-temperature applications, such as in nuclear reactors or aerospace components.
Vapor Pressure
The vapor pressure of zirconium at room temperature is negligible, indicating its low tendency to vaporize under standard conditions. This property is important in applications where the material’s stability and containment are critical.
Solubility in Water
Zirconium is not soluble in water but is soluble in hot concentrated acid. This characteristic is relevant in various chemical processes and industrial applications where the material’s reactivity and corrosion resistance are essential.
Practical Applications and Implications
The unique properties of zirconium, including its density, make it a valuable material in numerous industries.
Nuclear Engineering
In nuclear engineering, zirconium is widely used as a cladding material for nuclear fuel rods due to its low neutron absorption cross-section and resistance to corrosion by water and steam. The density of zirconium is a crucial factor in determining the structural integrity and performance of these fuel rods.
Material Science
Zirconium’s high density, strength, and corrosion resistance make it a popular choice for various engineering applications, such as in the production of cutting tools, abrasives, and refractory materials. The material’s density is a key consideration in these applications, as it affects the overall weight and mechanical properties of the final product.
Chemical Processes
The polar surface of zirconium, with its strong Lewis acid sites, makes it useful in various chemical reactions and processes. The material’s density and other quantifiable data, such as its solubility, are important factors in the design and optimization of these chemical systems.
Conclusion
The density of zirconium is a critical property that plays a crucial role in numerous industries, from nuclear engineering to material science and chemical processes. By understanding the factors that influence zirconium density, such as temperature, phase, atomic weight, and crystalline structure, we can better optimize its use and performance in various applications.
This comprehensive guide has provided a detailed overview of zirconium density, including the relevant measurable and quantifiable data, as well as the practical implications of this property. By leveraging this knowledge, researchers, engineers, and industry professionals can make informed decisions and develop innovative solutions that harness the full potential of this versatile material.
References:
- CHEMICAL THERMODYNAMICS OF ZIRCONIUM, https://www.oecd-nea.org/dbtdb/pubs/vol8-zirconium.pdf
- Zirconium in modern analytical chemistry – De Gruyter, https://www.degruyter.com/document/doi/10.1515/revac-2017-0016/html?lang=en
- Density and viscosity of liquid ZrO2 measured by aerodynamic levitation, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6658727/
- Zirconium Atom – an overview | ScienceDirect Topics, https://www.sciencedirect.com/topics/chemistry/zirconium-atom
- Provisional Peer-Reviewed Toxicity Values for Soluble Zirconium, https://cfpub.epa.gov/ncea/pprtv/documents/Zirconium.pdf

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.