Methanol, also known as wood alcohol, is a widely used chemical compound with diverse applications in various industries. One of the critical properties of methanol is its viscosity, which plays a crucial role in understanding its behavior and interactions in different systems. This comprehensive guide will delve into the intricacies of methanol’s viscosity, providing physics students with a detailed and technical understanding of this important property.
Viscosity of Methanol at Different Temperatures
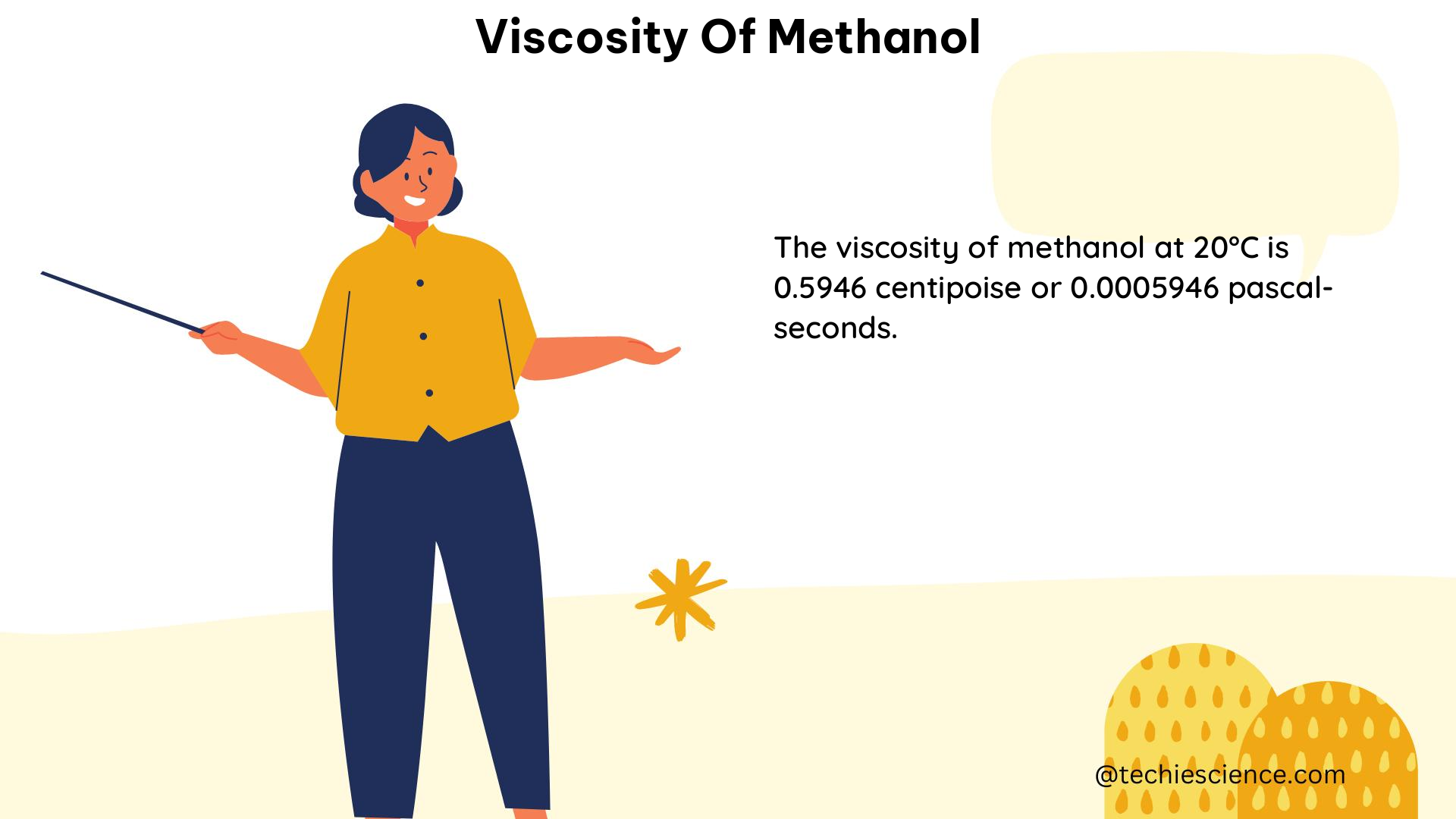
The viscosity of methanol is highly dependent on temperature, and understanding this relationship is essential for many applications. Here are the specific details on the viscosity of methanol at various temperatures:
| Temperature | Dynamic Viscosity (mPa·s) | Density (g/cm³) |
|---|---|---|
| 0°C | 0.797 | 0.8100 |
| 20°C | 0.594 | 0.7920 |
| 25°C | 0.543 | 0.7870 |
| 30°C | 0.507 | – |
| 50°C | 0.392 | – |
| 75°C | 0.294 | – |
| 100°C | 0.227 | – |
The viscosity of methanol decreases as the temperature increases, following the general trend of decreasing viscosity with rising temperature for most liquids. This behavior can be explained by the kinetic theory of gases, which states that as the temperature increases, the average kinetic energy of the molecules increases, leading to a reduction in the intermolecular forces and, consequently, a decrease in viscosity.
The relationship between the viscosity (η) and temperature (T) of methanol can be described by the Arrhenius equation:
η = A * e^(B/T)
Where:
– η is the dynamic viscosity of methanol (in mPa·s)
– T is the absolute temperature (in Kelvin)
– A and B are empirical constants specific to methanol, which can be determined experimentally.
By fitting the experimental data to the Arrhenius equation, the values of the constants A and B can be obtained, allowing for the accurate prediction of methanol’s viscosity at any given temperature within the studied range.
Viscosity of Methanol at Ultrahigh Pressures
In addition to the temperature dependence, the viscosity of methanol also varies significantly under the influence of high pressures. This is particularly important in applications where methanol is subjected to extreme pressure conditions, such as in the oil and gas industry or in certain chemical processes.
At atmospheric pressure, the viscosity of pure methanol is approximately 0.56 cP (centipoise). However, as the pressure increases, the viscosity of methanol-water mixtures can undergo significant changes:
- At 3500 bar (ultrahigh pressure), the viscosity of methanol-water mixtures increases, with the maximum viscosity shift occurring from around 55% organic at atmospheric pressure to 65% organic at 3500 bar.
This pressure-induced viscosity change can be attributed to the compression of the liquid and the resulting increase in the intermolecular interactions between the methanol and water molecules. The specific details of this relationship are crucial for understanding the behavior of methanol-based systems under high-pressure conditions.
Viscosity of Methanol-Water Mixtures
The viscosity of methanol is also influenced by the presence of other substances, such as water. The viscosity of methanol-water mixtures can be significantly different from that of pure methanol, and understanding this behavior is essential for various applications, including chemical processing, fuel blending, and separation techniques.
Here are the viscosity values for different methanol-water mixture compositions:
| Methanol Concentration | Viscosity (cP) |
|---|---|
| 20% Methanol | 1.36 |
| 40% Methanol | 1.64 |
| 60% Methanol | 1.55 |
| 80% Methanol | 1.19 |
| 100% Methanol | 0.5656 |
As the methanol concentration increases, the viscosity of the mixture initially increases, reaching a maximum around 40-60% methanol, and then decreases as the methanol content approaches 100%. This behavior is due to the complex interactions between the methanol and water molecules, which can lead to the formation of hydrogen bonds and other intermolecular forces that affect the overall viscosity of the mixture.
Understanding the viscosity of methanol-water mixtures is crucial in applications such as fuel blending, where the viscosity of the fuel mixture can impact engine performance and fuel delivery systems. It is also important in separation processes, where the viscosity of the mixture can affect the efficiency of techniques like distillation or extraction.
Advanced Correlation for Methanol Viscosity
To accurately model the viscosity of methanol over a wide range of conditions, including vapor, liquid, and supercritical states, a new reference-quality correlation has been developed by researchers. This advanced correlation incorporates various theoretical concepts to provide a comprehensive and accurate representation of methanol’s viscosity behavior.
The key features of this advanced correlation include:
-
Residual Concept: The correlation uses an advanced residual concept, which takes into account the deviations from the ideal gas behavior of methanol, to capture the viscosity changes across different fluid states.
-
Kinetic Theory: The correlation incorporates the principles of kinetic theory, which describe the relationship between the molecular properties of methanol and its viscosity.
-
Rainwater-Friend Theory: The Rainwater-Friend theory, which accounts for the effects of molecular interactions on viscosity, is also integrated into the correlation.
-
Enskog Dense Hard-Sphere Theory: The Enskog dense hard-sphere theory, which models the viscosity of dense fluids, is used to accurately represent the viscosity of methanol in the supercritical region.
By combining these advanced theoretical concepts, the new correlation provides a robust and reliable tool for predicting the viscosity of methanol under a wide range of conditions, making it invaluable for researchers and engineers working with methanol-based systems.
Measurement Techniques for Methanol Viscosity
To determine the viscosity of methanol and methanol-water mixtures, various measurement techniques have been developed and employed. Two of the most commonly used methods are:
- Ostwald Viscometer:
- The Ostwald viscometer is a simple and widely used instrument for measuring the viscosity of liquids, including methanol and methanol-water mixtures.
- The principle of the Ostwald viscometer is based on the time it takes for a fixed volume of the liquid to flow through a calibrated capillary under the influence of gravity.
-
By comparing the flow time of the reference fluid (typically water) with the flow time of the methanol or methanol-water mixture, the viscosity of the sample can be calculated.
-
Capillary Time-of-Flight (CTOF) Viscometer:
- The CTOF viscometer is a specialized instrument developed to measure the viscosity of solutions, including methanol-water mixtures, at ultrahigh pressures.
- This technique utilizes a capillary tube and measures the time-of-flight of the liquid as it flows through the capillary under the influence of a pressure differential.
- The CTOF viscometer is capable of measuring viscosities up to 3500 bar, making it a valuable tool for studying the behavior of methanol-based systems under extreme pressure conditions.
These measurement techniques, along with the advanced theoretical models, provide a comprehensive understanding of the viscosity of methanol and its behavior in various environments, enabling researchers and engineers to optimize the performance and design of methanol-based systems.
Conclusion
The viscosity of methanol is a critical property that varies significantly with temperature and pressure, as well as the presence of other substances, such as water. This comprehensive guide has provided physics students with a detailed and technical understanding of the intricacies of methanol’s viscosity, including the specific data, theoretical models, and measurement techniques used to characterize this important property.
By mastering the concepts and data presented in this guide, physics students will be better equipped to understand and predict the behavior of methanol-based systems, paving the way for innovative applications and advancements in various fields, from chemical processing to energy production.
References
- Xiang, H. W., Poling, B. E., & Prausnitz, J. M. (2019). A New Reference Correlation for the Viscosity of Methanol. Journal of Physical and Chemical Reference Data, 48(2), 023101.
- McGuire, P. (2012). Viscosity of Liquids: Methanol and Water. Woodlawn School.
- Anton Paar. Viscosity of Methanol.
- Dey, S. S., Ghosh, P., & Sarkar, R. (2006). Viscosity measurements of methanol–water and acetonitrile–water mixtures at ultrahigh pressures. Journal of Chromatography A, 1133(1-2), 141-148.
- Dey, S. S., Ghosh, P., & Sarkar, R. (2006). Viscosity measurements of methanol-water and acetonitrile-water mixtures at ultrahigh pressures. PubMed.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.