The viscosity of glycerol is a fundamental physical property that has been extensively studied in the scientific literature. Glycerol, also known as glycerin, is a colorless, odorless, and viscous liquid that is widely used in various industries, including pharmaceuticals, cosmetics, and food production. Understanding the viscosity of glycerol is crucial for many applications, as it affects the flow behavior, mixing, and processing of glycerol-based products.
Understanding Viscosity
Viscosity is a measure of a fluid’s resistance to flow. It is typically expressed in units of Poise (P) or Pascal-seconds (Pa·s), where 1 P = 0.1 Pa·s. The viscosity of a fluid depends on various factors, including temperature, pressure, and molecular structure.
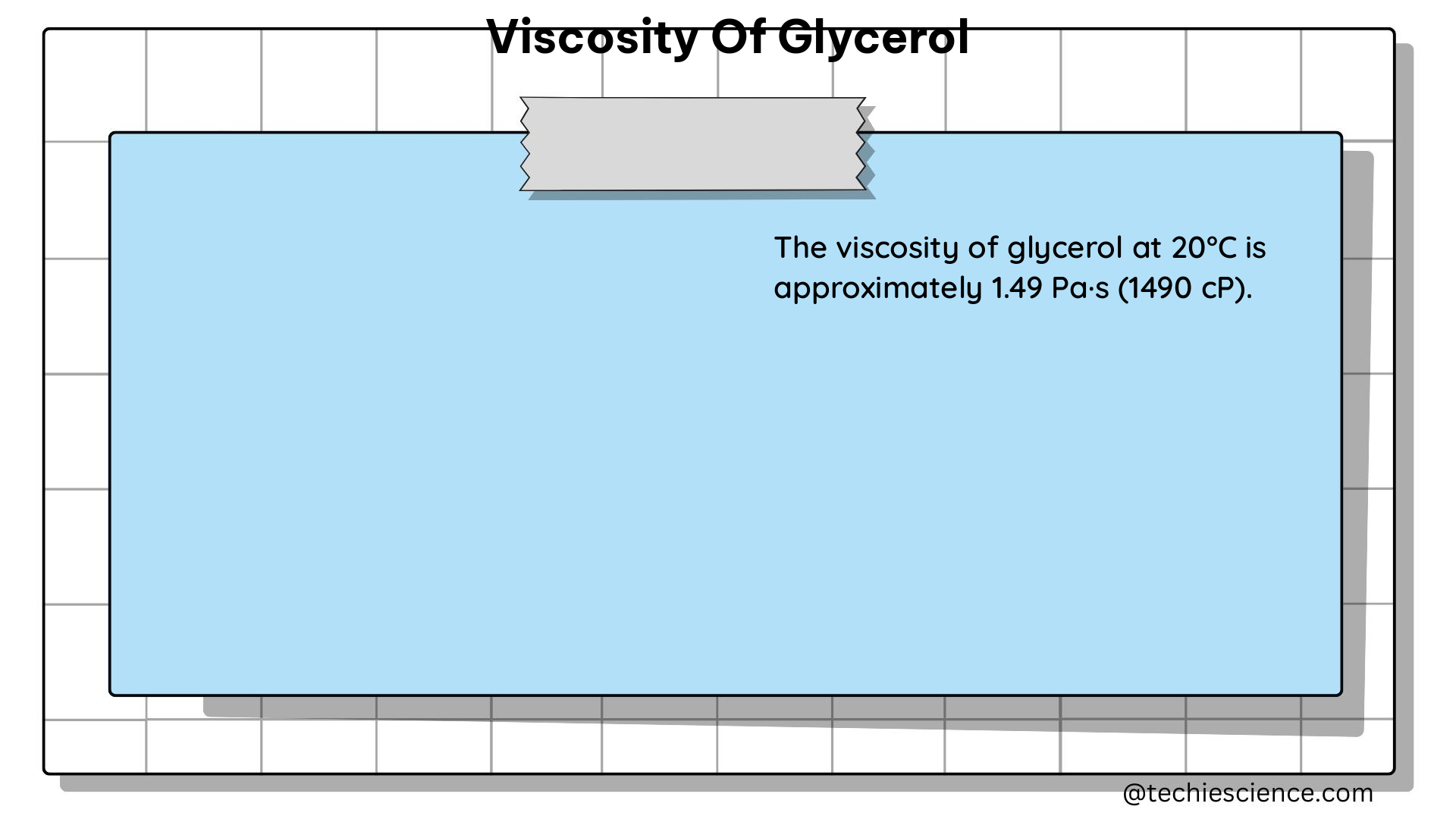
At room temperature (25°C), the viscosity of glycerol is approximately 1.5 Pa·s, which is over 1,000 times greater than the viscosity of water (0.001 Pa·s). This high viscosity is due to the strong intermolecular hydrogen bonding between glycerol molecules, which creates a dense and highly structured liquid.
Calculating Viscosity using Stokes’ Law
One common method for calculating the viscosity of glycerol is to use Stokes’ Law, which relates the viscosity of a fluid to the terminal velocity of a sphere falling through it. The equation for Stokes’ Law is as follows:
μ = ((2gr²)(d₁-d₂)/V) / 9
Where:
– μ = viscosity of the medium (dyne·s/cm²)
– g = acceleration due to gravity (cm/s²)
– r = radius of the sphere (cm)
– d₁ = density of the sphere (g/cm³)
– d₂ = density of the medium (g/cm³)
– V = terminal velocity of the sphere (cm/s)
Using the data provided in the first search result, we can calculate the viscosity of glycerol as follows:
μ = ((2*980*0.8²)(7.62-1.26) / 23) / 9
μ = 38.54 dynes
However, this value is not consistent with the given value for the viscosity of glycerol in the data book (0.942 N·s/m²). This discrepancy could be due to errors in the data or calculations, or it could be due to the use of different units or equations.
Experimental Techniques for Measuring Viscosity
To ensure accurate measurements of the viscosity of glycerol, it is important to use appropriate experimental techniques and equipment. One common method for measuring the viscosity of glycerol is to use a falling ball viscometer, which measures the time it takes for a ball bearing to fall through a known distance in the glycerol.
The steps for using a falling ball viscometer to measure the viscosity of glycerol are as follows:
- Prepare a sample of glycerol and place it in the viscometer.
- Measure the radius of the ball bearing and the distance it falls.
- Measure the time it takes for the ball bearing to fall through the known distance.
- Use Stokes’ Law to calculate the viscosity of the glycerol.
By measuring the radius of the ball bearing and the distance it falls, and using Stokes’ Law, the viscosity of the glycerol can be calculated. This method can provide accurate measurements of the viscosity of glycerol, but it is important to ensure that the experimental conditions are carefully controlled to minimize sources of error.
Theoretical Models for Predicting Viscosity
In addition to experimental measurements, the viscosity of glycerol can also be predicted using theoretical models and simulations. One example is the “Theoretical and Experimental Models on Viscosity: I…. Glycerol” paper, which discusses various models for predicting the viscosity of glycerol based on its molecular structure and interactions.
These theoretical models can be used to predict the viscosity of glycerol under a wide range of conditions, including different temperatures and pressures. By understanding the underlying physics and chemistry of glycerol, these models can provide valuable insights into the factors that influence its viscosity.
Factors Affecting Viscosity of Glycerol
The viscosity of glycerol can be influenced by several factors, including:
-
Temperature: The viscosity of glycerol decreases as the temperature increases. This is due to the weakening of the intermolecular hydrogen bonds between glycerol molecules, which reduces the resistance to flow.
-
Pressure: The viscosity of glycerol increases slightly with increasing pressure, as the molecules are compressed and the intermolecular distances are reduced.
-
Molecular Structure: The viscosity of glycerol is influenced by its molecular structure, which includes three hydroxyl (-OH) groups. These hydroxyl groups participate in strong hydrogen bonding, contributing to the high viscosity of glycerol.
-
Impurities: The presence of impurities, such as water or other substances, can affect the viscosity of glycerol. Impurities can disrupt the intermolecular interactions and alter the flow behavior of the liquid.
-
Shear Rate: The viscosity of glycerol can also depend on the shear rate, with the fluid exhibiting non-Newtonian behavior at low shear rates. This is due to the formation of transient molecular structures that can be disrupted by the applied shear.
Viscosity Data and Tables
Numerous studies have been conducted to measure the viscosity of glycerol under various conditions. Some of the key viscosity data and tables for glycerol include:
-
Glycerol Viscosity Tables: The American Chemical Society (ACS) has published viscosity tables for glycerol, providing data on the viscosity of glycerol at different temperatures and concentrations.
-
Viscosity Data at Low Rotational Velocities: A study published in the ResearchGate database provides viscosity data for glycerol at very low rotational velocities (0.01 rad/s), which can be useful for understanding the non-Newtonian behavior of glycerol.
-
Viscosity-Temperature Relationships: The ScienceDirect website hosts a study that explores the relationship between the viscosity and temperature of glycerol, providing empirical models and data for predicting the viscosity of glycerol over a wide range of temperatures.
These data sources and tables can be valuable resources for researchers, engineers, and industry professionals working with glycerol-based products, as they provide reliable and comprehensive information on the viscosity of this important fluid.
Conclusion
The viscosity of glycerol is a fundamental physical property that has been extensively studied and characterized in the scientific literature. Understanding the factors that influence the viscosity of glycerol, as well as the experimental techniques and theoretical models used to measure and predict it, is crucial for many applications involving this versatile liquid.
By leveraging the wealth of data and information available on the viscosity of glycerol, researchers and practitioners can optimize the performance, processing, and handling of glycerol-based products, leading to improved efficiency, quality, and safety in a wide range of industries.
References
- Viscosity Help: Calculating Glycerol Viscosity w/ Data, Physics Forums, 2005-02-26, https://www.physicsforums.com/threads/viscosity-help-calculating-glycerol-viscosity-w-data.65146/
- Measurement of the viscosity of glycerol – schoolphysics, https://www.schoolphysics.co.uk/age16-19/Properties%20of%20matter/Viscosity/text/Viscosity_of_glycerol_measurement/index.html
- Glycerol Viscosity Tables – ACS Publications, https://pubs.acs.org/doi/pdf/10.1021/ie50273a022?src=recsys
- Viscosity data of glycerol at very low rotational velocity (0.01 rad/s …, ResearchGate, 2019-04-16, https://www.researchgate.net/post/Viscosity-data-of-glycerol-at-very-low-rotational-velocity-001-rad-s-are-deviating-sharply-from-higher-ones-Is-there-a-way-to-stabilise-the-data
- The viscosity of glycerol – ScienceDirect.com, https://www.sciencedirect.com/science/article/abs/pii/S0021961417301817

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.