Glycerin, also known as glycerol, is a colorless, odorless, and viscous liquid with a wide range of applications in various industries, including pharmaceuticals, cosmetics, and food processing. The viscosity of glycerin is a critical property that varies with temperature, concentration, and purity, making it an essential parameter to understand for physics students and researchers. In this comprehensive blog post, we will delve into the intricacies of glycerin viscosity, providing a wealth of technical details and practical insights to help you master this topic.
Understanding Glycerin Viscosity
Viscosity is a measure of a fluid’s resistance to flow, and it plays a crucial role in the behavior and performance of glycerin in various applications. The viscosity of glycerin is influenced by several factors, including temperature, concentration, and purity, which we will explore in detail.
Viscosity of Pure Glycerin
The viscosity of pure glycerin, also known as anhydrous glycerin, can be measured at different temperatures. Here are the values for some common temperatures:
| Temperature | Viscosity (Pa·s) | Viscosity (cP) |
|---|---|---|
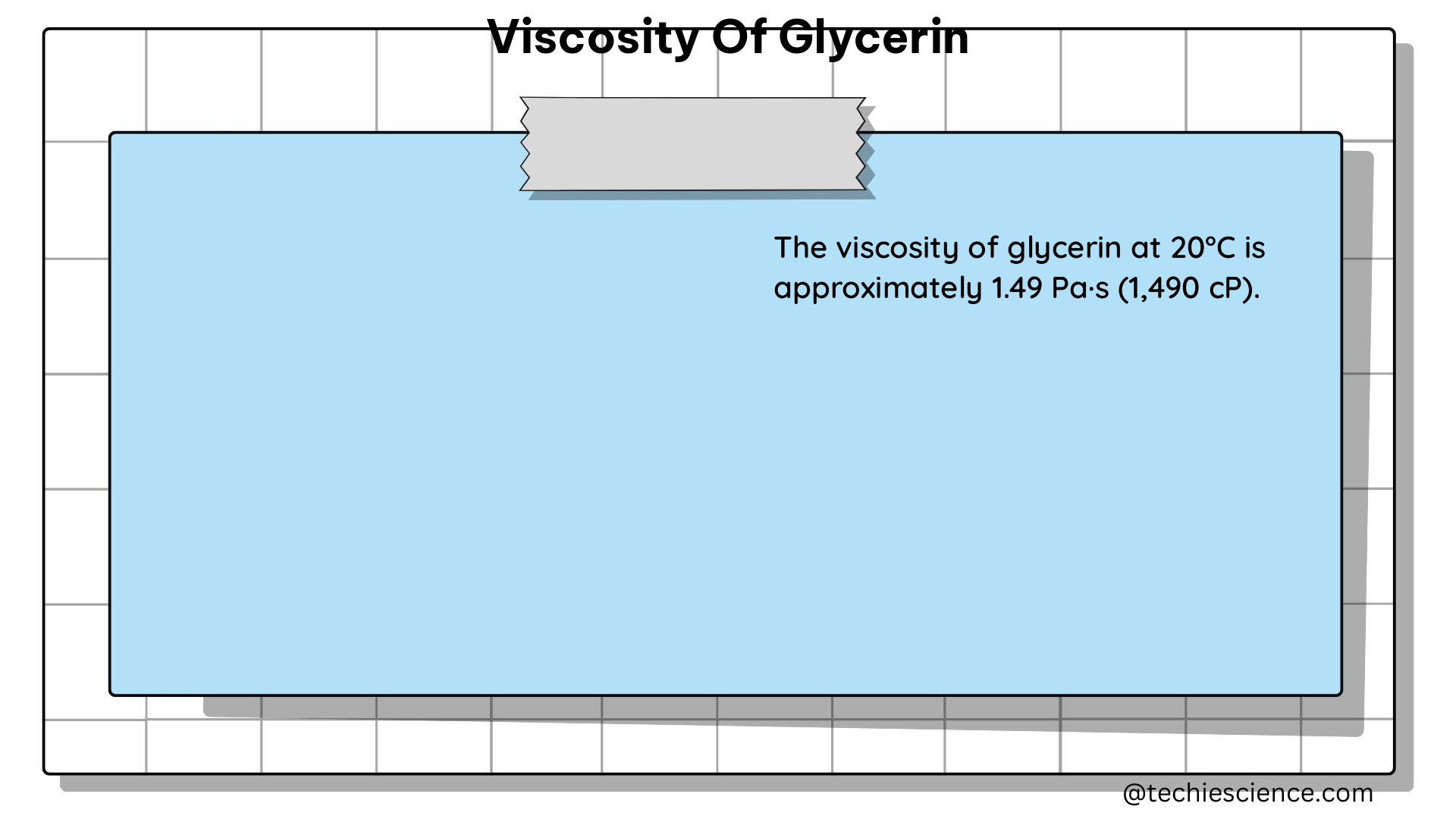
| 20°C | 870 | 870,000 |
| 25°C | 1,000 | 1,000,000 |
| 30°C | 1,200 | 1,200,000 |
| 40°C | 1,700 | 1,700,000 |
These values demonstrate the significant impact of temperature on the viscosity of pure glycerin. As the temperature increases, the viscosity decreases, a phenomenon known as the temperature-viscosity relationship.
Viscosity of Glycerin-Water Mixtures
The viscosity of glycerin-water mixtures also varies with the concentration of glycerin. Here are some examples of the viscosity at different glycerin concentrations at 20°C:
| Glycerin Concentration | Viscosity (Pa·s) | Viscosity (cP) |
|---|---|---|
| 100% (pure glycerin) | 870 | 870,000 |
| 50% | 1,200 | 1,200,000 |
| 25% | 1,700 | 1,700,000 |
| 10% | 2,500 | 2,500,000 |
As the glycerin concentration increases, the viscosity of the mixture also increases, reflecting the higher resistance to flow.
Viscosity of Aqueous Solutions of Glycerol
Glycerol, the chemical name for glycerin, can also be dissolved in water to form aqueous solutions. The viscosity of these solutions varies with the glycerol concentration, as shown in the following examples at 25°C:
| Glycerol Concentration | Viscosity (Pa·s) | Viscosity (cP) |
|---|---|---|
| 30% | 2.09 | 2,090 |
| 50% | 5.11 | 5,110 |
These values demonstrate the significant impact of glycerol concentration on the viscosity of aqueous solutions.
Temperature and Concentration Effects
The viscosity of glycerin is strongly influenced by both temperature and concentration, as we have seen in the previous sections.
Temperature Effect
As the temperature increases, the viscosity of glycerin decreases. This is due to the increased kinetic energy of the molecules, which reduces the intermolecular forces and allows the fluid to flow more easily. This relationship can be described by the Arrhenius equation, which relates the viscosity to the absolute temperature:
η = A * e^(B/T)
Where:
– η is the viscosity (Pa·s)
– A and B are constants specific to the fluid
– T is the absolute temperature (K)
This equation allows you to calculate the viscosity of glycerin at any given temperature, provided you have the appropriate constants for the specific glycerin sample.
Concentration Effect
As the concentration of glycerin increases, the viscosity also increases. This is due to the higher number of glycerin molecules in the solution, which results in stronger intermolecular interactions and a higher resistance to flow. The relationship between glycerin concentration and viscosity can be approximated by the following equation:
η = η₀ * (1 + 2.5φ + 10.05φ²)
Where:
– η is the viscosity of the glycerin-water mixture (Pa·s)
– η₀ is the viscosity of the pure solvent (water) (Pa·s)
– φ is the volume fraction of glycerin in the mixture
This equation provides a good estimate of the viscosity of glycerin-water mixtures, but it may not be accurate for very high or very low glycerin concentrations.
Purity Effects
The viscosity of glycerin can also be affected by its purity. Generally, higher purity glycerin has a lower viscosity, while lower purity glycerin has a higher viscosity. Here are some typical values for the viscosity of glycerin at 20°C, based on purity:
| Glycerin Purity | Viscosity (Pa·s) | Viscosity (cP) |
|---|---|---|
| High | 870 | 870,000 |
| Medium | 1,000 – 1,500 | 1,000,000 – 1,500,000 |
| Low | 1,500 – 2,000 | 1,500,000 – 2,000,000 |
The presence of impurities in glycerin can affect its molecular structure and intermolecular interactions, leading to changes in the overall viscosity.
Practical Applications and Considerations
The viscosity of glycerin is a critical parameter in various applications, including:
-
Pharmaceutical and Cosmetic Industries: Glycerin is widely used in the formulation of creams, lotions, and other personal care products. The viscosity of glycerin affects the texture, spreadability, and stability of these products.
-
Food Processing: Glycerin is used as a humectant, sweetener, and texturizer in various food products. The viscosity of glycerin influences the mouthfeel, consistency, and shelf-life of these products.
-
Chemical and Industrial Applications: Glycerin is used as a lubricant, plasticizer, and solvent in various industrial processes. The viscosity of glycerin affects its performance in these applications.
-
Experimental and Research Applications: Glycerin is often used as a model fluid in experimental studies, such as fluid dynamics and heat transfer experiments. The accurate knowledge of glycerin viscosity is essential for the interpretation and analysis of these experiments.
When working with glycerin, it is important to consider the effects of temperature, concentration, and purity on its viscosity. Proper measurement and control of these parameters can ensure the optimal performance and reliability of glycerin-based products and processes.
Conclusion
The viscosity of glycerin is a complex and multifaceted property that is crucial for understanding the behavior and performance of this versatile liquid. By delving into the technical details and practical applications presented in this blog post, you now have a comprehensive understanding of glycerin viscosity and the factors that influence it. Armed with this knowledge, you can confidently apply your expertise in various physics-related fields, from experimental design to product development and quality control.
References
- adityax26. (2018). Viscosity & Temperature – Linearisation and glycerine? Physics Forums. https://www.physicsforums.com/threads/viscosity-temperature-linearisation-and-glycerine.944785/
- Rion. (2024). Glycerin Viscosity Measurement: Impact of Temperature and Concentration. VT Rion Online Shop. https://vt.rion-onlineshop.com/blogs/technical-articles/glycerin-viscosity
- NCBI. (2022). Fluid Viscosity Measurement by Means of Secondary Flow in a Curved Microchannel. National Center for Biotechnology Information. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9502554/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.