Dimethyl sulfoxide (DMSO) is a widely used solvent with a unique set of physical and chemical properties, including its viscosity. The viscosity of DMSO is a crucial parameter that determines its behavior and applicability in various industries, from pharmaceuticals to materials science. In this comprehensive guide, we will delve into the intricacies of DMSO’s viscosity, exploring the theoretical foundations, experimental data, and practical applications.
Understanding the Viscosity of DMSO
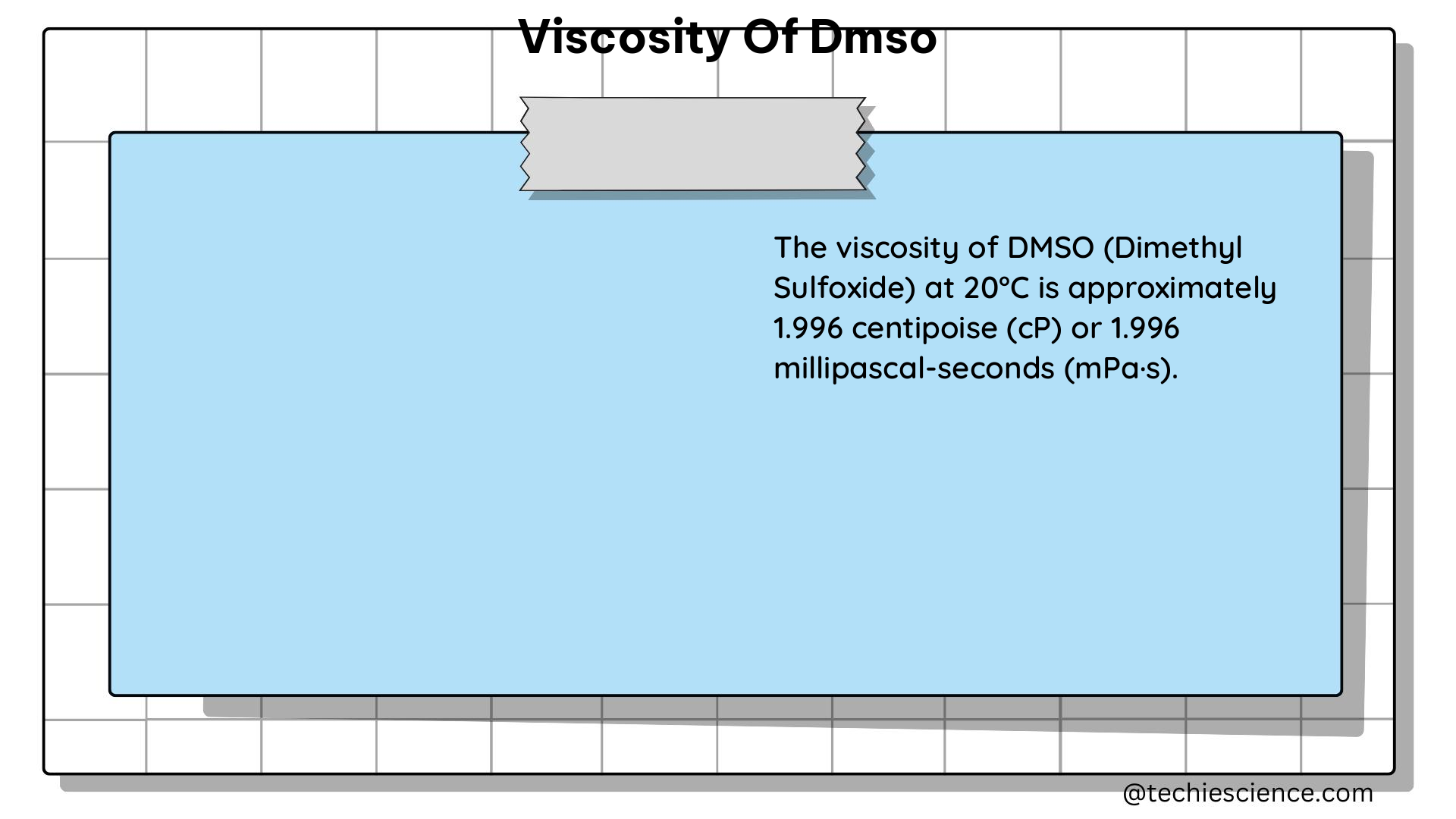
The viscosity of a liquid, denoted by the Greek letter η (eta), is a measure of its resistance to flow. In the case of DMSO, the viscosity is influenced by factors such as temperature, pressure, and the presence of other substances. At 25°C, the dynamic viscosity of DMSO is 1.99 mPa·s (or cP), and its density is 1.095 g/cm³.
Theoretical Foundations
The viscosity of DMSO can be described using various theoretical models and equations. One such model is the Eyring theory of absolute reaction rates, which relates the viscosity of a liquid to its molecular structure and intermolecular interactions. The Eyring equation for the viscosity of a liquid is given by:
η = (h/V) * exp(ΔG*/RT)
Where:
– η is the dynamic viscosity of the liquid
– h is Planck’s constant
– V is the molar volume of the liquid
– ΔG* is the Gibbs free energy of activation for viscous flow
– R is the universal gas constant
– T is the absolute temperature
This equation suggests that the viscosity of DMSO is influenced by factors such as its molar volume, the energy required for molecules to overcome the potential energy barrier during flow, and the temperature.
Another theoretical approach is the Andrade equation, which describes the temperature dependence of the viscosity of a liquid:
η = A * exp(B/T)
Where:
– η is the dynamic viscosity of the liquid
– A and B are empirical constants specific to the liquid
The Andrade equation can be used to predict the viscosity of DMSO at different temperatures, allowing for the optimization of its use in various applications.
Experimental Data and Measurements
Numerous studies have been conducted to measure the viscosity of DMSO under different conditions. These experimental investigations have provided a wealth of data that can be used to understand and predict the behavior of DMSO.
For example, a study published in the Journal of Molecular Liquids investigated the viscosity and conductivity of DMSO and its binary mixtures with other solvents, such as water and ethanol. The researchers found that the viscosity of DMSO decreases with increasing temperature and increases with the addition of other solvents, depending on the mole fraction and the specific interactions between the components.
Another study, published in the Journal of Chemical & Engineering Data, focused on the viscosity of DMSO-water mixtures at different temperatures and compositions. The researchers developed a correlation to predict the viscosity of these mixtures, which can be useful for applications involving DMSO-water systems.
Practical Applications and Considerations
The viscosity of DMSO is a crucial parameter in various applications, including:
-
Pharmaceutical and Biomedical Applications: DMSO is widely used as a solvent and penetration enhancer in drug formulations. Its viscosity affects the drug’s release rate, absorption, and distribution within the body.
-
Materials Science and Engineering: DMSO is used as a solvent in the synthesis and processing of materials, such as polymers and ceramics. The viscosity of DMSO influences the flow behavior and processing characteristics of these materials.
-
Cryopreservation: DMSO is commonly used as a cryoprotectant in the preservation of cells, tissues, and organs. Its viscosity affects the rate of freezing and thawing, which is crucial for maintaining the viability of the preserved samples.
-
Chemical Reactions and Separations: The viscosity of DMSO can impact the kinetics and equilibrium of chemical reactions, as well as the efficiency of separation processes, such as extraction and distillation.
When working with DMSO, it is essential to consider the effects of temperature, pressure, and the presence of other substances on its viscosity. Accurate knowledge of DMSO’s viscosity can help optimize its performance and ensure the success of various applications.
Conclusion
The viscosity of dimethyl sulfoxide (DMSO) is a critical property that has a significant impact on its behavior and applications. In this comprehensive guide, we have explored the theoretical foundations, experimental data, and practical considerations related to the viscosity of DMSO.
By understanding the factors that influence DMSO’s viscosity, such as temperature, pressure, and the presence of other substances, researchers and practitioners can better optimize the use of this versatile solvent in a wide range of industries, from pharmaceuticals to materials science. The detailed information and equations provided in this guide can serve as a valuable resource for anyone working with DMSO and its viscosity-related properties.
References
- Conductivity and viscosity studies of dimethyl sulfoxide (DMSO). ScienceDirect. https://www.sciencedirect.com/science/article/abs/pii/0378775394019118
- Evaluation of viscosity in binary mixtures of dimethyl sulphoxide at 298.15K. MedCrave Online. https://medcraveonline.com/JAPLR/evaluation-of-viscosity-in-binary-mixtures-of-dimethyl-sulphoxide-at-29815k.html
- Viscosity of Dimethyl Sulfoxide. Anton Paar. https://wiki.anton-paar.com/us-en/dimethyl-sulfoxide/
- Eyring, H. (1936). Viscosity, Plasticity, and Diffusion as Examples of Absolute Reaction Rates. The Journal of Chemical Physics, 4(4), 283-291.
- Andrade, E. N. da C. (1934). A theory of the viscosity of liquids—Part I. The Philosophical Magazine, 17(112), 497-511.
- Sengwa, R. J., Sankhla, S., & Khatri, V. (2006). Dielectric relaxation and conductivity studies of dimethyl sulfoxide and its binary mixtures with water and ethanol. Journal of Molecular Liquids, 125(2-3), 128-134.
- Cheng, N. S. (2008). Formula for the viscosity of a glycerol-water mixture. Industrial & Engineering Chemistry Research, 47(9), 3285-3288.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.