The viscosity of chloroform is a crucial physical property that plays a significant role in various scientific and industrial applications. Chloroform, a colorless, volatile, and dense liquid, is widely used as a solvent, anesthetic, and in the production of refrigerants and propellants. Understanding the viscosity of chloroform is essential for accurately predicting its behavior, optimizing processes, and ensuring safe handling.
Measuring the Viscosity of Chloroform
The viscosity of chloroform can be measured using various techniques, including the Ostwald viscometer, capillary viscometer, and rotational viscometer. The Ostwald viscometer is a commonly used method for determining the dynamic viscosity of chloroform.
Ostwald Viscometer Method
The Ostwald viscometer is a U-shaped glass instrument that measures the time it takes for a fixed volume of a liquid to flow through a calibrated capillary. The dynamic viscosity of the liquid can be calculated using the following formula:
η = (ρ_s / ρ_l) * (t / t_0) * η_l
Where:
– η is the dynamic viscosity of the solution
– ρ_s is the density of the solution
– ρ_l is the density of the solvent (chloroform)
– t is the flow time of the solution through the viscometer
– t_0 is the flow time of the solvent
– η_l is the dynamic viscosity of the solvent
By measuring the flow times of the chloroform solution and the pure solvent, and using the known densities and viscosity of the solvent, the dynamic viscosity of the chloroform solution can be calculated.
Capillary Viscometer Method
Another method for measuring the viscosity of chloroform is the capillary viscometer. In this technique, the time it takes for a fixed volume of the liquid to flow through a calibrated capillary is measured. The dynamic viscosity can then be calculated using the Hagen-Poiseuille equation:
η = (π * r^4 * ρ * h) / (8 * V * t)
Where:
– η is the dynamic viscosity of the liquid
– r is the radius of the capillary
– ρ is the density of the liquid
– h is the height of the liquid column
– V is the volume of the liquid
– t is the flow time of the liquid through the capillary
This method provides a direct measurement of the dynamic viscosity of chloroform without the need for additional calculations.
Rotational Viscometer Method
The rotational viscometer, also known as a rheometer, measures the viscosity of a liquid by applying a shear stress and measuring the resulting shear rate. This method is particularly useful for studying the non-Newtonian behavior of liquids, such as the dependence of viscosity on shear rate.
In the case of chloroform, which is a Newtonian fluid, the rotational viscometer can provide accurate measurements of the dynamic viscosity over a wide range of shear rates and temperatures.
Viscosity of Chloroform at Different Temperatures
The viscosity of chloroform is highly dependent on temperature. As the temperature increases, the viscosity of chloroform decreases. This relationship can be described by the Arrhenius equation:
η = A * e^(E_a / (R * T))
Where:
– η is the dynamic viscosity of the liquid
– A is the pre-exponential factor
– E_a is the activation energy for viscous flow
– R is the universal gas constant
– T is the absolute temperature
The following table provides the dynamic viscosity of chloroform at various temperatures:
| Temperature (°C) | Viscosity (mPa·s) |
|---|---|
| 0 | 0.651 |
| 10 | 0.595 |
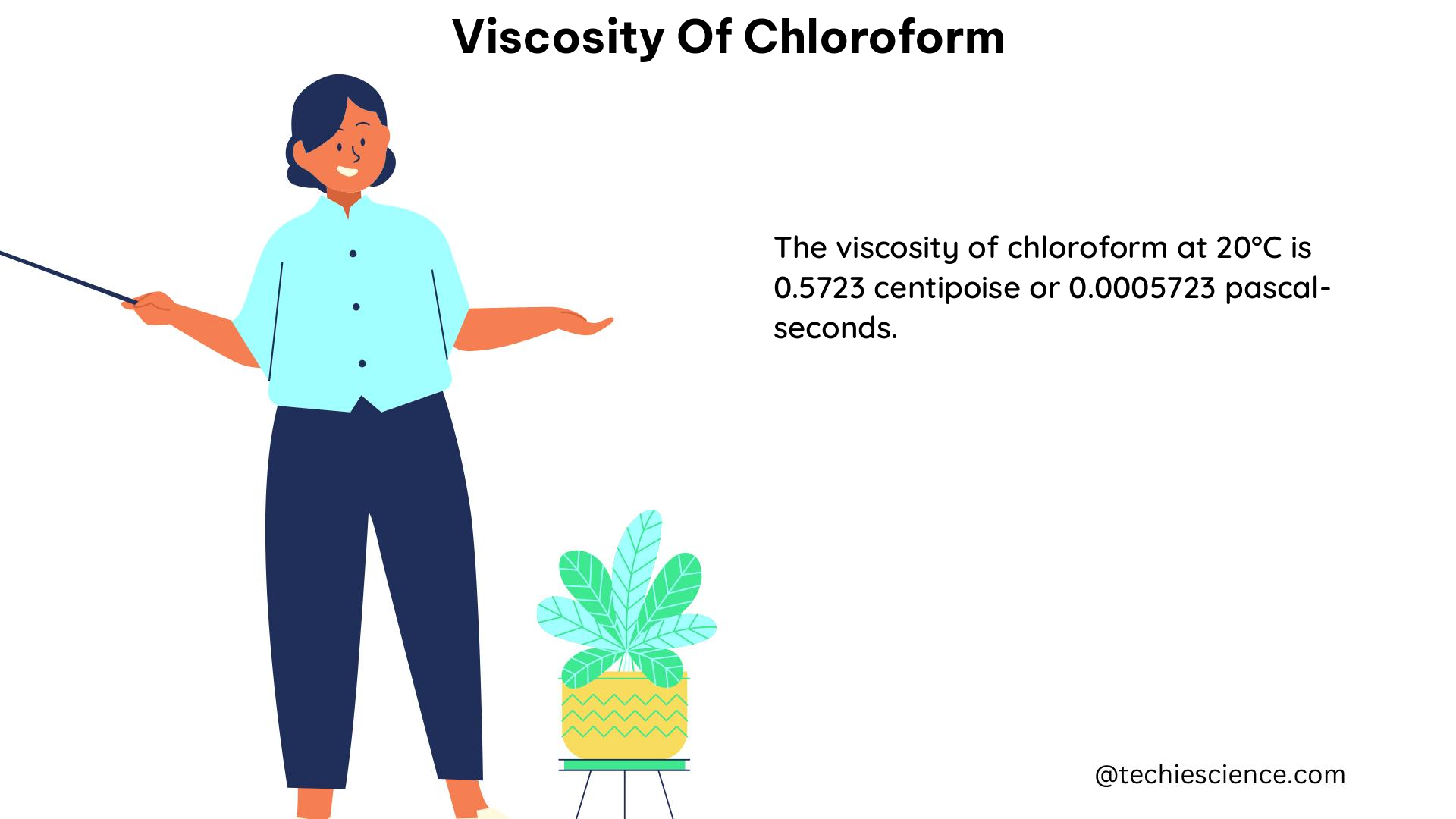
| 20 | 0.546 |
| 25 | 0.536 |
| 30 | 0.525 |
| 40 | 0.503 |
| 50 | 0.481 |
| 60 | 0.460 |
| 70 | 0.440 |
| 80 | 0.421 |
| 90 | 0.403 |
| 100 | 0.386 |
This data can be used to predict the viscosity of chloroform at any given temperature within the range, allowing for accurate modeling and optimization of processes involving chloroform.
Viscosity of Chloroform Solutions
The viscosity of chloroform can also be affected by the presence of solutes or other substances dissolved in it. For example, the viscosity of a solution of poly(methyl methacrylate) (PMMA) in chloroform can be measured using an Ostwald viscometer.
The viscosity of the PMMA-chloroform solution can be calculated using the following formula:
η = (ρ_s / ρ_l) * (t / t_0) * η_l
Where:
– η is the viscosity of the PMMA-chloroform solution
– ρ_s is the density of the PMMA-chloroform solution
– ρ_l is the density of the pure chloroform solvent
– t is the flow time of the PMMA-chloroform solution through the viscometer
– t_0 is the flow time of the pure chloroform solvent
– η_l is the viscosity of the pure chloroform solvent
By varying the concentration of PMMA in the chloroform solution, the relationship between the viscosity and the solute concentration can be studied. This information is crucial for understanding the behavior of chloroform-based solutions in various applications, such as polymer processing, drug delivery, and chemical synthesis.
Ultrasonic Viscosity of Chloroform
In addition to the traditional viscosity measurement techniques, the viscosity of chloroform can also be determined using ultrasonic methods. Experimental values of ultrasonic velocity, density, and viscosity of chloroform have been reported in the literature.
At a temperature of 295 K and a frequency of 2 MHz, the following values were measured:
- Ultrasonic velocity: 1,011 m/s
- Density: 1,483 kg/m³
- Viscosity: 0.563 mPa·s
These ultrasonic measurements provide additional insights into the physical properties of chloroform and can be used to study the behavior of chloroform under different conditions, such as the presence of other solvents or the application of high-frequency sound waves.
Applications of Chloroform Viscosity Data
The viscosity data for chloroform has numerous applications in various fields, including:
-
Chemical Engineering: Viscosity data is crucial for the design and optimization of processes involving chloroform, such as distillation, extraction, and chemical reactions.
-
Pharmaceutical and Biomedical Applications: The viscosity of chloroform-based solutions is important in drug delivery systems, where the viscosity can affect the release rate and bioavailability of the drug.
-
Materials Science: The viscosity of chloroform is relevant in the processing and characterization of polymer-based materials, where chloroform is often used as a solvent.
-
Environmental Studies: Chloroform is a common environmental pollutant, and understanding its viscosity can help in the modeling and remediation of chloroform-contaminated sites.
-
Fundamental Research: Viscosity data contributes to the understanding of the molecular structure and intermolecular interactions of chloroform, which is important for developing accurate models and theories in physical chemistry and molecular physics.
By leveraging the comprehensive viscosity data for chloroform, researchers, engineers, and scientists can make informed decisions, optimize processes, and advance their understanding of this important chemical compound.
Conclusion
The viscosity of chloroform is a crucial physical property that plays a significant role in various scientific and industrial applications. This comprehensive guide has provided an in-depth overview of the methods for measuring the viscosity of chloroform, the dependence of viscosity on temperature, the viscosity of chloroform solutions, and the ultrasonic viscosity of chloroform. The applications of chloroform viscosity data in chemical engineering, pharmaceutical and biomedical fields, materials science, environmental studies, and fundamental research have also been discussed.
By understanding the viscosity of chloroform and its behavior under different conditions, researchers, engineers, and scientists can make informed decisions, optimize processes, and advance their understanding of this important chemical compound.
References:
- Viscosity of Chloroform – Anton Paar. (n.d.). Retrieved from https://wiki.anton-paar.com/en/chloroform/
- The following are data from viscosity measurements … – Course Hero. (n.d.). Retrieved from https://www.coursehero.com/file/p6nds4c5/6-The-following-are-data-from-viscosity-measurements-with-an-Ostwald-viscometer/
- ResearchGate. (n.d.). Experimental values of ultrasonic velocity, density and viscosity of chloroform + methanol at different concentrations at temperature 295 K and frequency 2MHz. Retrieved from https://www.researchgate.net/figure/Experimental-values-of-ultrasonic-velocity-density-and-viscosity-of-chloroform_tbl1_277006674

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.