The viscosity of benzene, a widely used hydrocarbon solvent, is a crucial physical property that plays a significant role in various applications in chemistry, engineering, and materials science. Understanding the factors that influence the viscosity of benzene, as well as the experimental data and correlations available, is essential for physics students and researchers working in these fields.
Understanding the Viscosity of Benzene
Viscosity is a measure of a fluid’s resistance to flow, and it is a fundamental property that affects the behavior of liquids and gases. The viscosity of benzene can be expressed in two forms: dynamic (absolute) viscosity and kinematic viscosity.
Dynamic (Absolute) Viscosity
The dynamic viscosity of benzene, denoted by the symbol μ, is a measure of its resistance to shear stress or flow. It is typically expressed in units of centipoise (cP) or millipascal-seconds (mPa·s). The dynamic viscosity of benzene decreases with increasing temperature and increases with increasing pressure.
The relationship between the dynamic viscosity of benzene and temperature can be expressed using the following empirical equation:
μ = A * exp(B/T)
where:
– μ is the dynamic viscosity of benzene in mPa·s
– T is the absolute temperature in Kelvin (K)
– A and B are empirical constants that depend on the specific fluid and can be determined experimentally
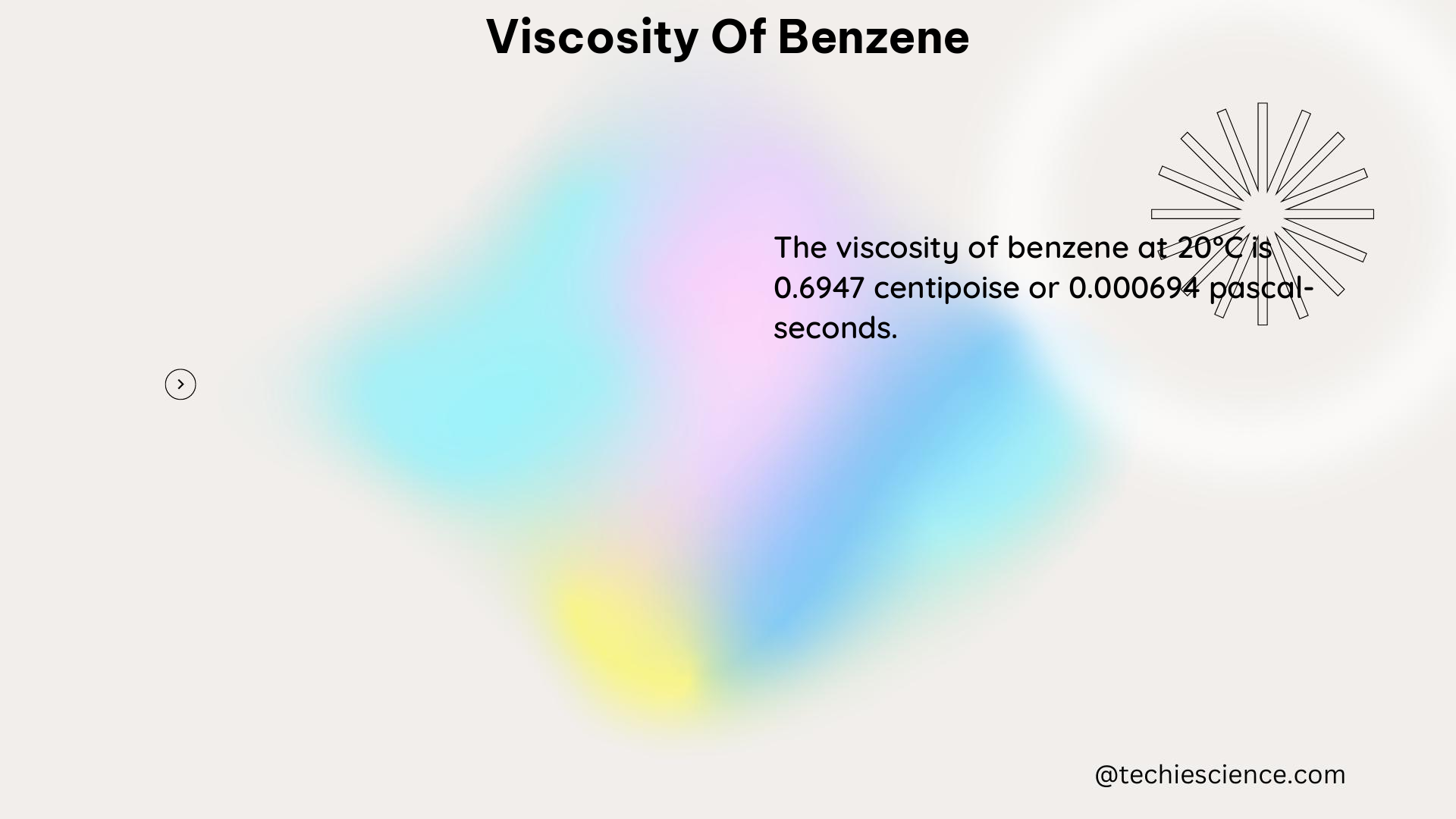
According to the Engineering Toolbox, the dynamic viscosity of benzene at atmospheric pressure and 20°C is about 0.603 mPa·s, while at 40°C it is about 0.456 mPa·s, and at 60°C it is about 0.361 mPa·s. The dynamic viscosity of benzene at higher pressures and temperatures can be estimated using empirical correlations or experimental data.
Kinematic Viscosity
The kinematic viscosity of benzene, denoted by the symbol ν, is a measure of its resistance to flow relative to its density. It is typically expressed in units of centistokes (cSt) or square millimeters per second (mm²/s). The kinematic viscosity of benzene can be calculated by dividing its dynamic viscosity by its density, as shown in the following equation:
ν = μ / ρ
where:
– ν is the kinematic viscosity of benzene in cSt or mm²/s
– μ is the dynamic viscosity of benzene in mPa·s
– ρ is the density of benzene in g/cm³
According to the Engineering Toolbox, the kinematic viscosity of benzene at atmospheric pressure and 20°C is about 0.706 cSt, while at 40°C it is about 0.530 cSt, and at 60°C it is about 0.410 cSt.
Factors Affecting the Viscosity of Benzene
The viscosity of benzene, like that of other fluids, is influenced by various factors, including temperature and pressure.
Temperature Dependence
The viscosity of benzene decreases with increasing temperature. This is due to the increased kinetic energy of the molecules, which reduces the intermolecular forces and allows the molecules to flow more easily. The relationship between the dynamic viscosity of benzene and temperature can be described by the Arrhenius equation:
μ = A * exp(B/T)
where A and B are empirical constants that can be determined experimentally.
Pressure Dependence
The viscosity of benzene increases with increasing pressure. This is because higher pressures lead to a decrease in the intermolecular distances, which in turn increases the intermolecular forces and the resistance to flow. The effect of pressure on the viscosity of benzene is more pronounced at higher temperatures and pressures.
For example, the viscosity of benzene at 50°C and 10 MPa is about 0.406 mPa·s, while at 100°C and 10 MPa it is about 0.281 mPa·s.
Experimental Data and Correlations
Numerous experimental studies and correlations have been reported in the literature for the viscosity of benzene as a function of temperature, pressure, and composition. Some of the key references include:
- International Journal of Thermophysics (1991): Measurements of the viscosity of benzene, toluene, and m-xylene at pressures up to 80 MPa and temperatures up to 473 K.
- Journal of Chemical & Engineering Data (2009): Viscosity and density data for five hydrocarbon liquids, including benzene, at pressures up to 200 MPa and temperatures up to 473 K.
- Journal of Physical and Chemical Reference Data (2014): A reference correlation for the viscosity of benzene from the triple point to 675 K and up to 300 MPa.
These studies provide valuable experimental data and correlations that can be used to estimate the viscosity of benzene under various conditions.
Practical Applications of Benzene Viscosity
The viscosity of benzene is an important property that has numerous applications in chemistry, engineering, and materials science, including:
- Fluid Flow: The viscosity of benzene affects the flow behavior of the fluid, which is crucial in the design of piping systems, pumps, and other fluid handling equipment.
- Heat Transfer: The viscosity of benzene influences the heat transfer characteristics of the fluid, which is important in the design of heat exchangers and other thermal systems.
- Rheology: The viscosity of benzene is a key parameter in the study of the rheological properties of materials, which is important in the development of coatings, adhesives, and other polymer-based products.
- Solvent Applications: The viscosity of benzene affects its ability to dissolve and transport solutes, which is important in various chemical processes and applications.
Understanding the viscosity of benzene and its dependence on temperature, pressure, and other factors is crucial for physics students and researchers working in these fields.
Conclusion
The viscosity of benzene is a well-studied and important physical property that has been extensively investigated by researchers and organizations. This comprehensive guide has provided an in-depth understanding of the dynamic and kinematic viscosities of benzene, the factors that influence them, and the experimental data and correlations available in the literature. By mastering the concepts and applications presented in this guide, physics students and researchers can better understand and predict the behavior of benzene in various applications, ultimately contributing to advancements in chemistry, engineering, and materials science.
References
- Measurements of the viscosity of benzene, toluene, and m-xylene at pressure up to 80 MPa, International Journal of Thermophysics, 1991.
- Viscosity and Density of Five Hydrocarbon Liquids at Pressures up to 200 MPa and Temperatures up to 473 K, Journal of Chemical & Engineering Data, 2009.
- Reference Correlation of the Viscosity of Benzene from the Triple Point to 675 K and up to 300 MPa, Journal of Physical and Chemical Reference Data, 2014.
- Engineering Toolbox, Benzene – Viscosity as a Function of Temperature and Pressure, https://www.engineeringtoolbox.com/benzene-viscosity-temperature-pressure-d_1479.html

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.