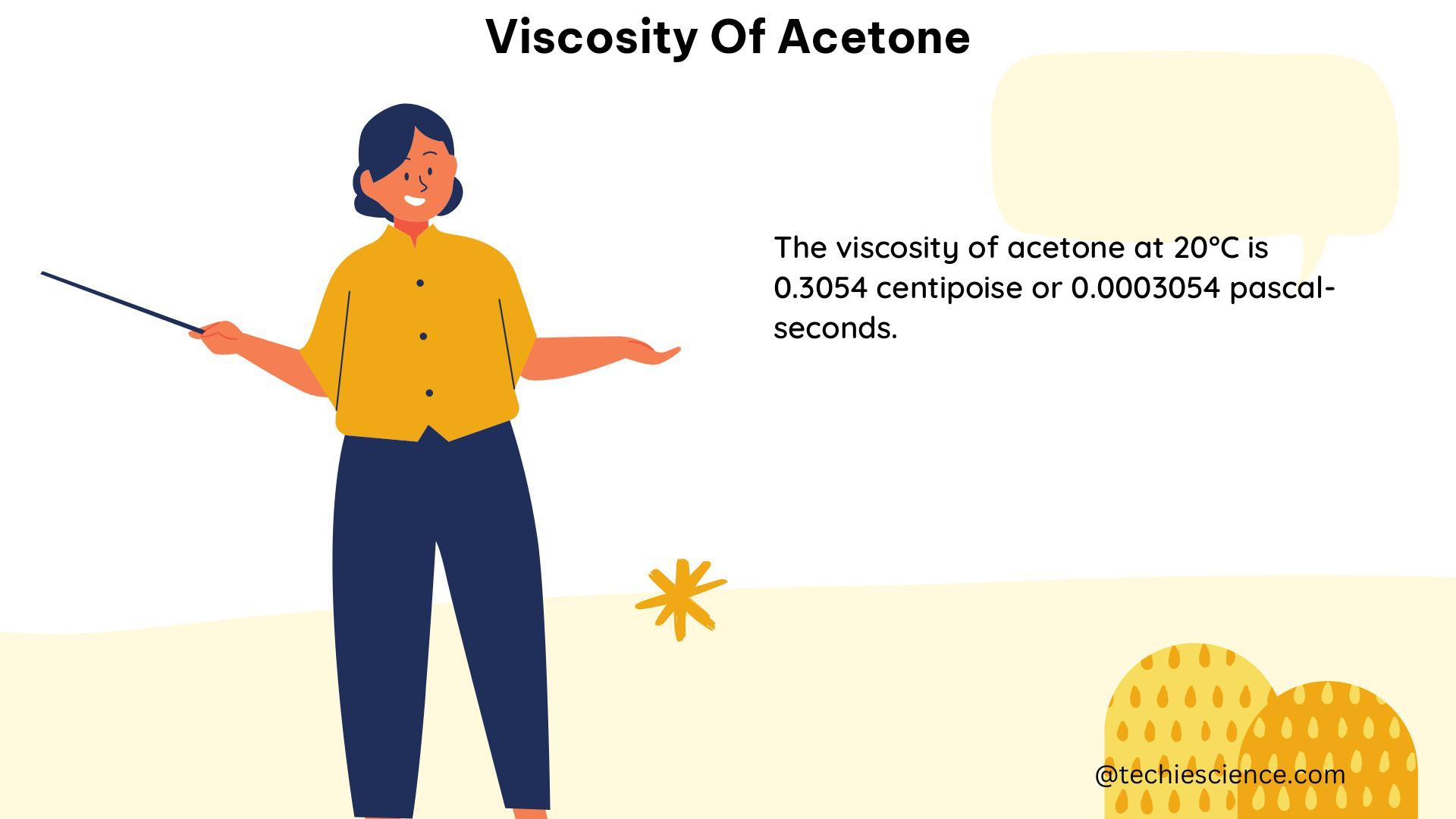
The viscosity of acetone, a widely used organic solvent, is a crucial property that varies with temperature. This comprehensive guide delves into the technical details and specific data points related to the viscosity of acetone, providing a valuable resource for physics students.
Understanding Viscosity and Its Importance
Viscosity is a fundamental property of fluids that measures a fluid’s resistance to flow. It is a quantitative measure of the internal friction within a fluid, which determines the fluid’s ability to resist deformation under shear stress. Viscosity is an essential parameter in various fields, including fluid mechanics, heat transfer, and chemical engineering.
The viscosity of a fluid can be classified into two types:
- Dynamic Viscosity: This represents the internal friction of a fluid and is measured in units of Pascal-seconds (Pa·s) or millipascal-seconds (mPa·s).
- Kinematic Viscosity: This is the ratio of dynamic viscosity to the fluid’s density and is measured in units of square meters per second (m²/s).
Understanding the viscosity of acetone is crucial in applications such as:
– Chemical processing and synthesis
– Solvent-based extraction and purification
– Coatings and adhesives formulation
– Pharmaceutical and cosmetic product development
Viscosity of Acetone: Temperature Dependence
The viscosity of acetone is strongly influenced by temperature, as shown in the viscosity table provided by Anton Paar:
| Temperature (°C) | Dynamic Viscosity (mPa·s) |
|---|---|
| 0 | 0.40 |
| 30 | 0.30 |
| 50 | 0.25 |
| 75 | 0.20 |
| 100 | 0.17 |
These values, sourced from the Kaye & Laby reference, demonstrate that as the temperature of acetone increases, its dynamic viscosity decreases. This relationship can be described by the Arrhenius equation, which relates the viscosity of a fluid to its absolute temperature:
η = A * e^(E_a / (R * T))
Where:
– η is the dynamic viscosity of the fluid (in this case, acetone)
– A is a pre-exponential factor
– E_a is the activation energy for viscous flow
– R is the universal gas constant
– T is the absolute temperature
By fitting experimental data to this equation, the values of the pre-exponential factor and activation energy can be determined, allowing for accurate prediction of acetone’s viscosity at different temperatures.
Viscosity of Acetone-Based Systems
In addition to the temperature-dependent viscosity of pure acetone, research has been conducted on the viscosity of acetone-based systems, such as:
Saturated Gas Expanded Liquid System of Acetone and Carbon Dioxide
A study published on ResearchGate provides experimental viscosity measurements for the saturated gas expanded liquid system of acetone and carbon dioxide. The researchers used a falling-weight method to determine the viscosities under various pressure conditions. This data is valuable for understanding the behavior of acetone-CO2 mixtures, which are commonly used in supercritical fluid extraction and other industrial processes.
Ternary System of Acetone-Ethanol-Water
A thesis presented in the NJIT Digital Commons explores the viscosity of a three-component system consisting of acetone, ethanol, and water at 25°C. The study includes a tabular and graphical comparison of literature and experimental values for the viscosity of this ternary system. This information is useful for applications involving acetone-based mixtures, such as in the formulation of coatings, adhesives, and pharmaceutical products.
Viscosity Measurement Techniques
The viscosity of acetone can be measured using various experimental techniques, including:
-
Capillary Viscometry: This method involves measuring the time it takes for a fixed volume of the fluid to flow through a calibrated capillary tube under the influence of gravity. The dynamic viscosity can then be calculated using the Hagen-Poiseuille equation.
-
Rotational Viscometry: In this technique, the fluid is sheared between a rotating bob and a stationary cup. The torque required to maintain a constant rotational speed is used to determine the dynamic viscosity.
-
Falling-Ball Viscometry: A dense ball is allowed to fall through the fluid, and the time it takes to fall a fixed distance is used to calculate the kinematic viscosity, which can then be converted to dynamic viscosity using the fluid’s density.
-
Vibrating-Plate Viscometry: This method measures the damping of a vibrating plate immersed in the fluid, which is related to the fluid’s viscosity.
The choice of measurement technique depends on factors such as the fluid’s properties, the desired accuracy, and the available equipment.
Viscosity Calculations and Examples
To illustrate the application of viscosity principles, let’s consider a few examples:
Example 1: Calculating the Viscous Force on a Sphere
Suppose a sphere with a radius of 2 mm is moving through acetone at 25°C with a velocity of 0.5 m/s. Given that the dynamic viscosity of acetone at 25°C is 0.31 mPa·s, calculate the viscous force acting on the sphere.
The viscous force on a sphere moving through a fluid can be calculated using the Stokes’ law equation:
F_v = 6 * π * η * r * v
Where:
– F_v is the viscous force (in Newtons)
– η is the dynamic viscosity of the fluid (in Pa·s)
– r is the radius of the sphere (in meters)
– v is the velocity of the sphere (in m/s)
Substituting the given values:
F_v = 6 * π * 0.31 × 10^-3 Pa·s * 0.002 m * 0.5 m/s
F_v = 1.85 × 10^-6 N
Therefore, the viscous force acting on the sphere moving through acetone at 25°C is approximately 1.85 × 10^-6 N.
Example 2: Determining the Terminal Velocity of a Sphere
Consider a sphere with a density of 2500 kg/m³ and a radius of 1 mm falling through acetone at 20°C. Given that the dynamic viscosity of acetone at 20°C is 0.33 mPa·s, calculate the terminal velocity of the sphere.
The terminal velocity of a sphere falling through a fluid can be calculated using the following equation:
v_t = √[(2 * g * (ρ_s - ρ_f) * r^2) / (9 * η)]
Where:
– v_t is the terminal velocity (in m/s)
– g is the acceleration due to gravity (9.8 m/s²)
– ρ_s is the density of the sphere (in kg/m³)
– ρ_f is the density of the fluid (in kg/m³)
– r is the radius of the sphere (in m)
– η is the dynamic viscosity of the fluid (in Pa·s)
Substituting the given values:
v_t = √[(2 * 9.8 m/s² * (2500 kg/m³ - 791 kg/m³) * (0.001 m)^2) / (9 * 0.33 × 10^-3 Pa·s)]
v_t = 0.0225 m/s
Therefore, the terminal velocity of the sphere falling through acetone at 20°C is approximately 0.0225 m/s.
These examples demonstrate how the viscosity of acetone can be used in various calculations and applications, such as determining the viscous force on an object and the terminal velocity of a falling sphere.
Conclusion
The viscosity of acetone is a crucial property that varies with temperature and plays a significant role in various applications. This comprehensive guide has provided detailed information on the temperature-dependent viscosity of acetone, the viscosity of acetone-based systems, and the techniques used to measure viscosity. Additionally, the provided examples illustrate how the viscosity of acetone can be applied in practical calculations and problem-solving for physics students.
By understanding the technical details and specific data points related to the viscosity of acetone, physics students can enhance their knowledge and problem-solving skills, ultimately preparing them for real-world applications in fields such as fluid mechanics, chemical engineering, and materials science.
References
- Viscosity measurements on saturated gas expanded liquid systems – Acetone and carbon dioxide. (n.d.). ResearchGate. Retrieved from https://www.researchgate.net/publication/244360433_Viscosity_measurements_on_saturated_gas_expanded_liquid_systems-Acetone_and_carbon_dioxide
- The surface tension and viscosity values for diethyl ether, acetone, and ethanol. (n.d.). YouTube. Retrieved from https://www.youtube.com/watch?v=P5hpuatB_eQ
- Viscosity – Formula, Measurement, & Equation – Lesson. (n.d.). Study.com. Retrieved from https://study.com/learn/lesson/viscosity-formula-measurement-equation.html
- Viscosity of a three component system, acetone-ethanol-water, at 25°C. (n.d.). NJIT Digital Commons. Retrieved from https://digitalcommons.njit.edu/cgi/viewcontent.cgi?article=2528&context=theses
- Viscosity of Acetone. (n.d.). Anton Paar. Retrieved from https://wiki.anton-paar.com/us-en/acetone/

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.