Valsartan, a widely prescribed angiotensin II receptor blocker (ARB), exhibits a complex and pH-dependent solubility profile that significantly impacts its bioavailability and pharmacokinetics. Understanding the intricacies of valsartan’s solubility is crucial for formulation development, drug delivery, and optimization of therapeutic outcomes.
Understanding the pH-Dependent Solubility of Valsartan
Valsartan’s solubility is highly dependent on the pH of the surrounding environment. In acidic conditions, such as the stomach (pH 1-3), valsartan exists primarily in its protonated form, which has a relatively low solubility. However, as the pH increases along the gastrointestinal (GI) tract, valsartan’s solubility dramatically improves due to the formation of its dianion salt.
Solubility in Phosphate Buffer (pH 8.0)
At a pH of 8.0, which is representative of the small intestine, valsartan’s solubility is significantly enhanced. In a phosphate buffer at 25°C, valsartan’s solubility reaches an impressive 16.8 g/L. This substantial increase in solubility is attributed to the formation of the dianion salt, which is more soluble than the protonated form.
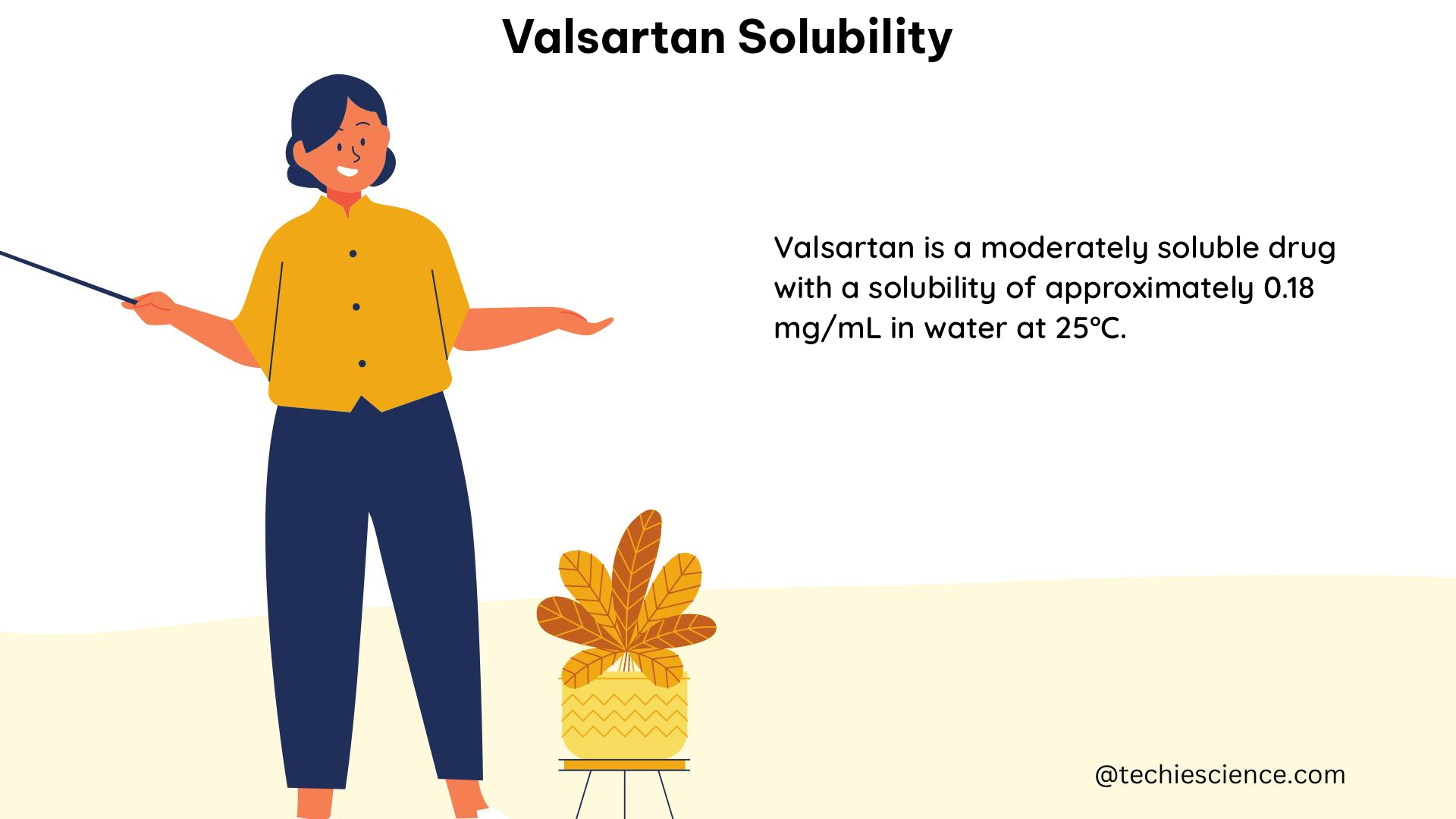
Solubility in Water
In contrast, valsartan’s solubility in water at 25°C is much lower, reaching only 0.18 g/L. This stark difference in solubility between the buffered solution and water highlights the critical role of pH in modulating valsartan’s solubility.
Interplay between Valsartan Solubility and Absorption Rate
Valsartan’s pH-dependent solubility also influences its absorption rate along the GI tract. As the pH increases from 6 to 7.5, the absorption rate of valsartan decreases, indicating an inverse relationship between solubility and absorption.
This phenomenon can be explained by the fact that as the pH increases, valsartan’s solubility increases, but the absorption rate decreases. This is likely due to the formation of the more soluble dianion salt, which may have a slower rate of permeation across the intestinal epithelium compared to the protonated form.
Valsartan Solubility in Formulation Development
The solubility of valsartan is a crucial consideration in the formulation and development of various dosage forms. Researchers have investigated the solubility of valsartan in different media to optimize its delivery and bioavailability.
Solubility in 0.1 N HCl, Methanol, and Phosphate Buffer (pH 6.8)
In the formulation and evaluation of valsartan solid dispersion, it was determined that valsartan was soluble in 0.1 N HCl, methanol, and a phosphate buffer at pH 6.8. These solvents were used to prepare the solid dispersion formulations, taking advantage of valsartan’s solubility in these media.
Saturation Solubility Studies
Saturation solubility studies have been conducted to quantify valsartan’s solubility in various media, including distilled water, 0.1 N HCl, and pH 6.8 phosphate buffer. These studies, employing the Higuchi and Connors technique, have provided valuable insights into the solubility profile of valsartan under different conditions.
Strategies to Enhance Valsartan Solubility
To address the challenges posed by valsartan’s pH-dependent solubility, researchers have explored various strategies to improve its solubility and, consequently, its bioavailability.
Cyclodextrin Complexation
The use of hydroxypropyl-β-cyclodextrin (HP-β-CD) has been investigated as a method to enhance the solubility and stability of valsartan. Cyclodextrin complexation can effectively increase the aqueous solubility of valsartan by forming inclusion complexes, which can subsequently improve its dissolution and absorption.
Solid Dispersion Formulation
The development of valsartan solid dispersion formulations has also been explored as a strategy to enhance its solubility and dissolution rate. By dispersing valsartan in a suitable carrier, such as polymers or surfactants, the surface area available for dissolution can be increased, leading to improved solubility and bioavailability.
Conclusion
Valsartan’s solubility is a complex and multifaceted phenomenon that plays a crucial role in its pharmacokinetics, formulation, and administration. Understanding the pH-dependent nature of valsartan’s solubility, its interrelation with absorption rate, and the strategies employed to enhance its solubility is essential for optimizing the therapeutic efficacy of this widely used ARB.
By delving into the technical details and specific data points surrounding valsartan solubility, this comprehensive guide aims to provide science students with a valuable resource for understanding the intricacies of this important pharmaceutical property.
References:
- BIOAVAILABILITY FILE: VALSARTAN. http://dergi.fabad.org.tr/pdf/volum32/issue4/185-196.pdf
- Formulation and evaluation of valsartan solid dispersion for improved dissolution rate. https://wjbphs.com/sites/default/files/WJBPHS-2023-0314.pdf
- Improvement of solubility and stability of valsartan by hydroxypropyl-bcyclodextrin. https://www.researchgate.net/publication/312656905_Improvement_of_solubility_and_stability_of_valsartan_by_hydroxypropyl-bcyclodextrin

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.