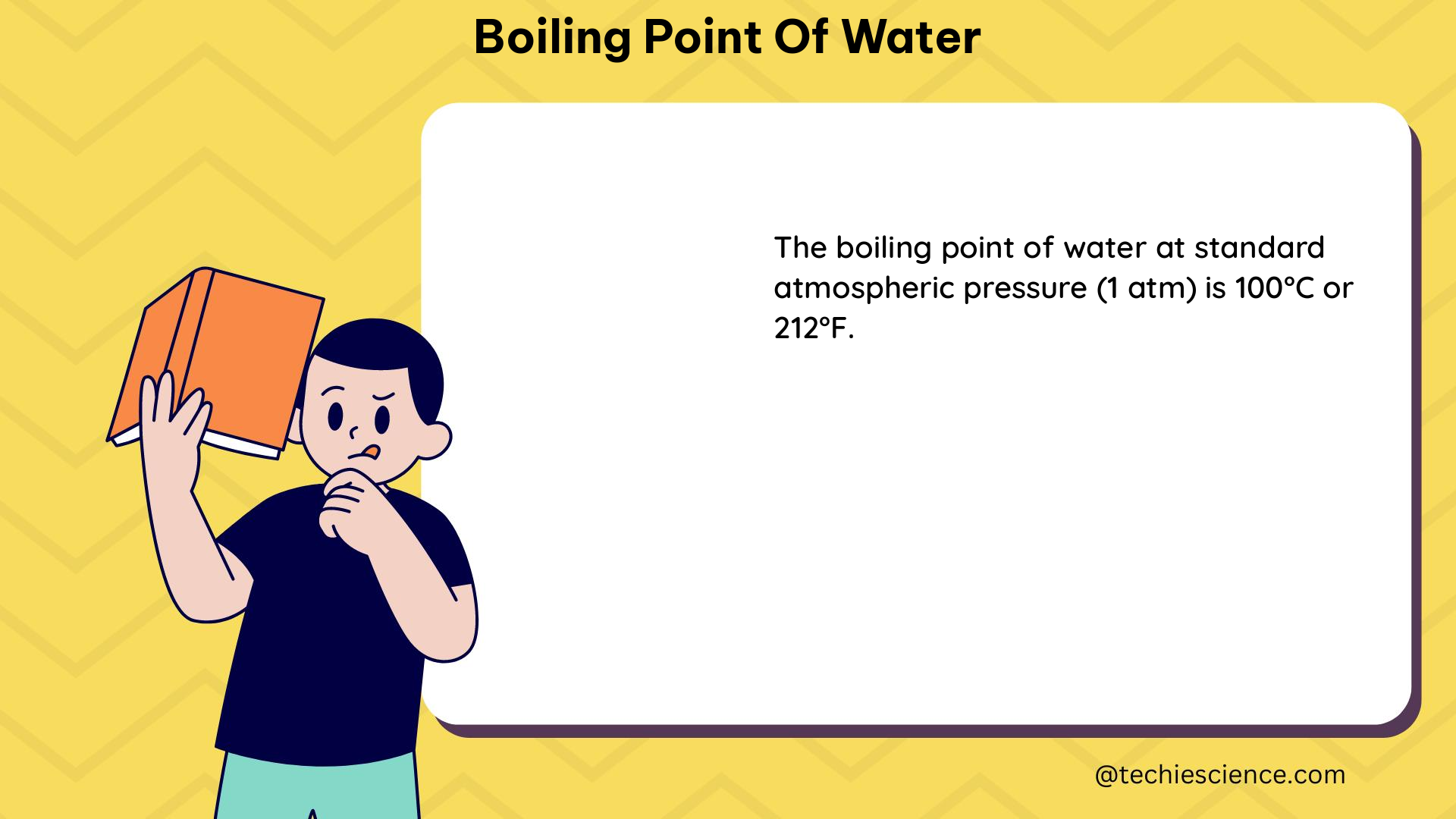
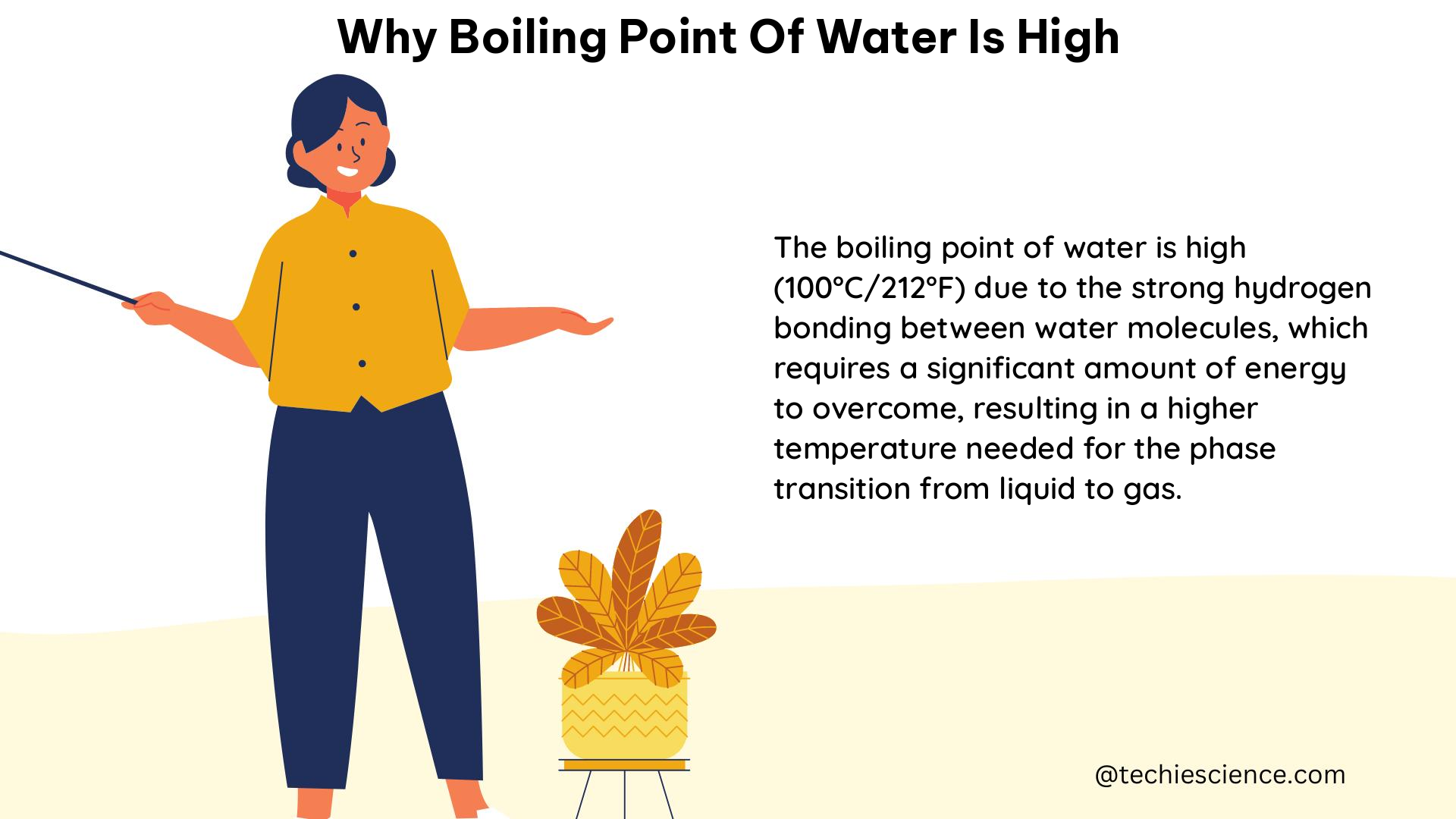
The boiling point of water is a crucial concept in physics and chemistry, as it plays a significant role in various processes and applications. The high boiling point of water, which is 100°C (212°F) at standard atmospheric pressure, is a result of several factors, including the presence of hydrogen bonds, the polar nature of the water molecule, and the high latent heat of vaporization.
The Role of Hydrogen Bonds in Water’s High Boiling Point
Water molecules are polar, with a partial negative charge on the oxygen atom and partial positive charges on the hydrogen atoms. This polarity allows for the formation of hydrogen bonds between water molecules, which are relatively strong intermolecular attractions. The energy required to break these hydrogen bonds is significant, and as a result, more heat energy is needed to convert liquid water into water vapor, leading to a higher boiling point.
The strength of the hydrogen bonds in water can be quantified using the following formula:
E_H = -2.5 × 10^-19 J/bond
where E_H represents the energy of a single hydrogen bond. This value indicates that the energy required to break a hydrogen bond in water is relatively high, contributing to the high boiling point.
The Influence of Water’s Polar Nature on Boiling Point
The polar nature of the water molecule is another crucial factor in determining its high boiling point. The partial charges on the oxygen and hydrogen atoms create a dipole moment, which results in strong intermolecular attractions between water molecules. These attractions, known as dipole-dipole interactions, also require a significant amount of energy to overcome, further contributing to the high boiling point of water.
The magnitude of the dipole moment in water can be calculated using the following formula:
μ = q × d
where μ is the dipole moment, q is the partial charge, and d is the distance between the partial charges. For water, the dipole moment is approximately 1.85 Debye, which is a relatively high value compared to other molecules.
The Impact of Latent Heat of Vaporization on Boiling Point
The latent heat of vaporization, which is the amount of energy required to convert a liquid into a gas, is another crucial factor in determining the boiling point of water. Water has a high latent heat of vaporization, which means that a significant amount of energy is needed to overcome the intermolecular forces and convert liquid water into water vapor.
The latent heat of vaporization for water can be calculated using the following formula:
L_v = 2.26 × 10^6 J/kg
This high value of the latent heat of vaporization contributes to the high boiling point of water, as more energy is required to overcome the intermolecular forces and transition the liquid into the gaseous state.
Experimental Observations and Variations in Boiling Point
Experimental data supports the theoretical explanations for the high boiling point of water. At standard atmospheric pressure (1 atm), the boiling point of water is 100°C (212°F). However, the boiling point of water can vary depending on the atmospheric pressure.
For example, at higher elevations where the atmospheric pressure is lower, the boiling point of water is also lower. This can be observed in the following table:
| Elevation (m) | Atmospheric Pressure (atm) | Boiling Point of Water (°C) |
|---|---|---|
| 0 | 1.000 | 100.0 |
| 1,000 | 0.899 | 93.4 |
| 2,000 | 0.805 | 90.0 |
| 3,000 | 0.718 | 86.6 |
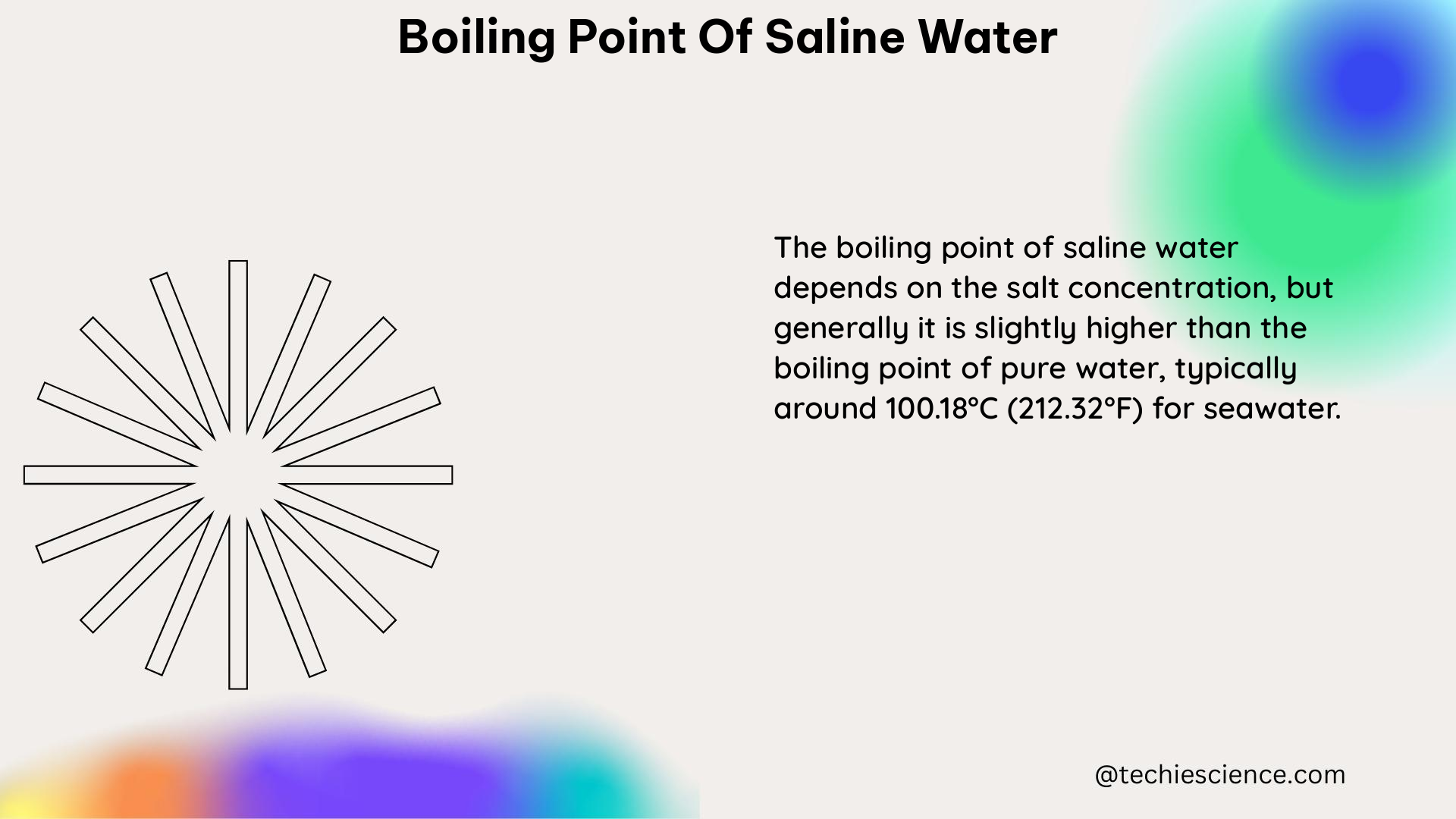
Additionally, the boiling point of water can be affected by the presence of solutes. When a solute is added to water, the boiling point of the solution is higher than the boiling point of pure water. This phenomenon, known as boiling point elevation, is a colligative property that depends on the number of particles in the solution rather than their identity.
The boiling point elevation constant for water is 0.512°C per mole of solute (0.900°F per mole of solute). For example, if a 1-molar (1 mol/L) NaCl solution is prepared, the boiling point of the solution will be 101.7°C (215.1°F), which is 1.7°C (3.1°F) higher than the boiling point of pure water. This is because the NaCl solute dissociates into two ions (Na+ and Cl-) in solution, increasing the number of particles and thus elevating the boiling point.
Conclusion
The high boiling point of water is a result of the complex interplay between the presence of hydrogen bonds, the polar nature of the water molecule, and the high latent heat of vaporization. These factors work together to require a significant amount of energy to convert liquid water into water vapor, leading to the observed high boiling point of 100°C (212°F) at standard atmospheric pressure.
Understanding the factors that contribute to water’s high boiling point is crucial in various fields, such as chemistry, physics, and engineering, where the behavior of water and its phase transitions are of paramount importance.
References:
– “Dependence of Boiling Point of Water on Pressure.” Physics Experiments, 3 Aug. 2022, physicsexperiments.eu/1707/dependence-of-boiling-point-of-water-on-pressure.
– “Quantitative structure‐property relationships for prediction of boiling points of organic compounds.” Environmental Toxicology and Chemistry, vol. 28, no. 11, 2009, pp. 2320-2327, doi: 10.1897/01-363.
– “Freezing Point Depression and Boiling Point Elevation.” Chemistry LibreTexts, chem.libretexts.org/Courses/College_of_Marin/CHEM_114:_Introductory_Chemistry/13:_Solutions/13.09:_Freezing_Point_Depression_and_Boiling_Point_Elevation-_Making_Water_Freeze_Colder_and_Boil_Hotter.