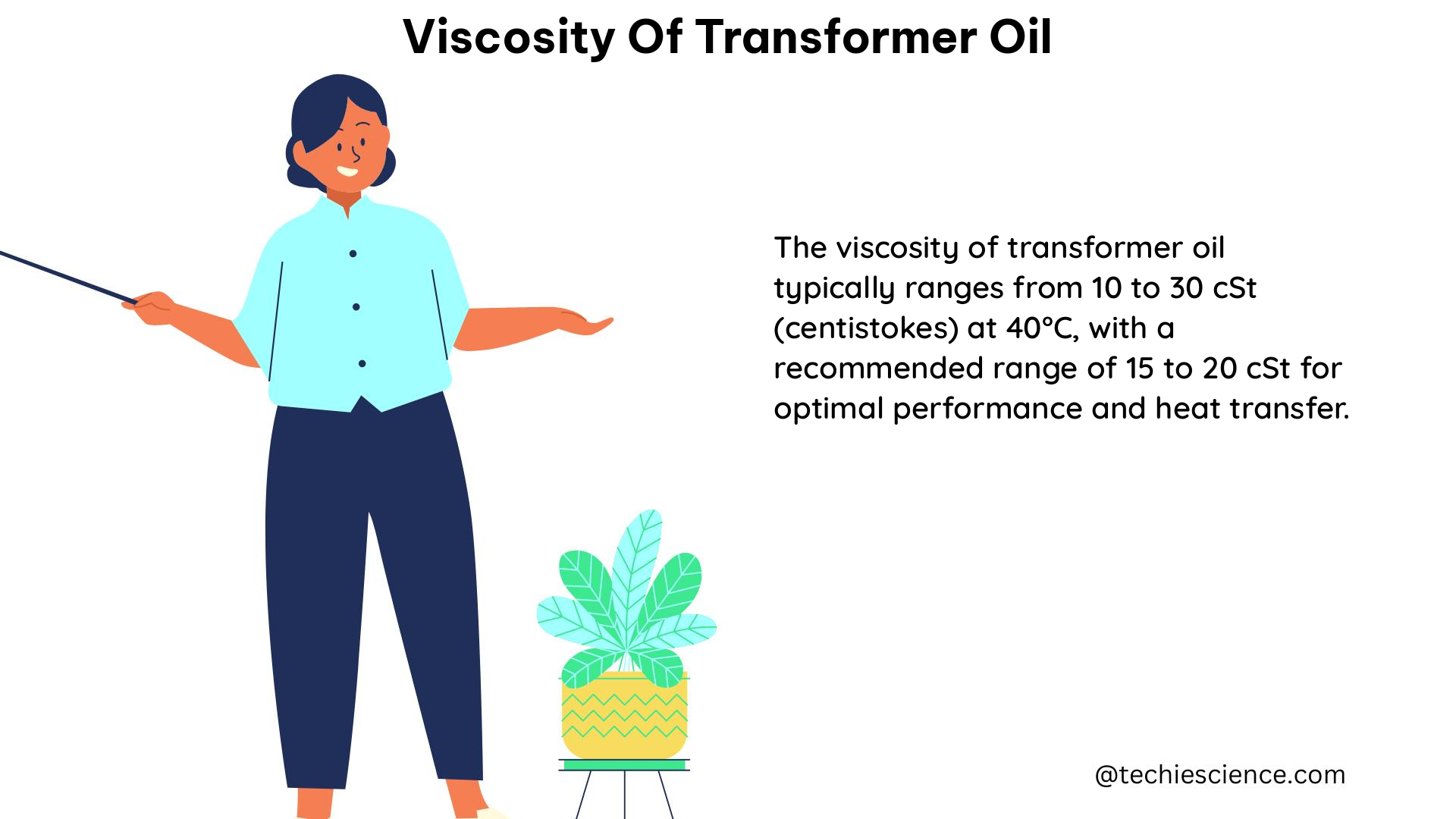
Boiling point is a crucial property in various fields of science and engineering, and understanding whether it is an intensive or extensive property is essential. In this comprehensive guide, we will delve into the intricacies of boiling point, its relationship with intensive and extensive properties, and provide a detailed exploration of the topic.
Understanding Intensive and Extensive Properties
Intensive properties are characteristics of a substance that do not depend on the amount of the substance present. These properties remain the same regardless of the quantity of the substance. Examples of intensive properties include temperature, pressure, density, and boiling point.
On the other hand, extensive properties are characteristics that depend on the amount of the substance present. These properties are additive, meaning that the total property of a system is the sum of the properties of its parts. Examples of extensive properties include mass, volume, and energy content.
Is Boiling Point an Intensive Property?

The boiling point of a substance is an intensive property, which means it does not depend on the amount of the substance present. This can be demonstrated through the following examples and explanations:
Theorem: Boiling Point is an Intensive Property
The boiling point of a substance is an intensive property because it is a characteristic of the substance that remains the same regardless of the quantity of the substance present. This can be mathematically expressed as:
Boiling Point = f(P, T)
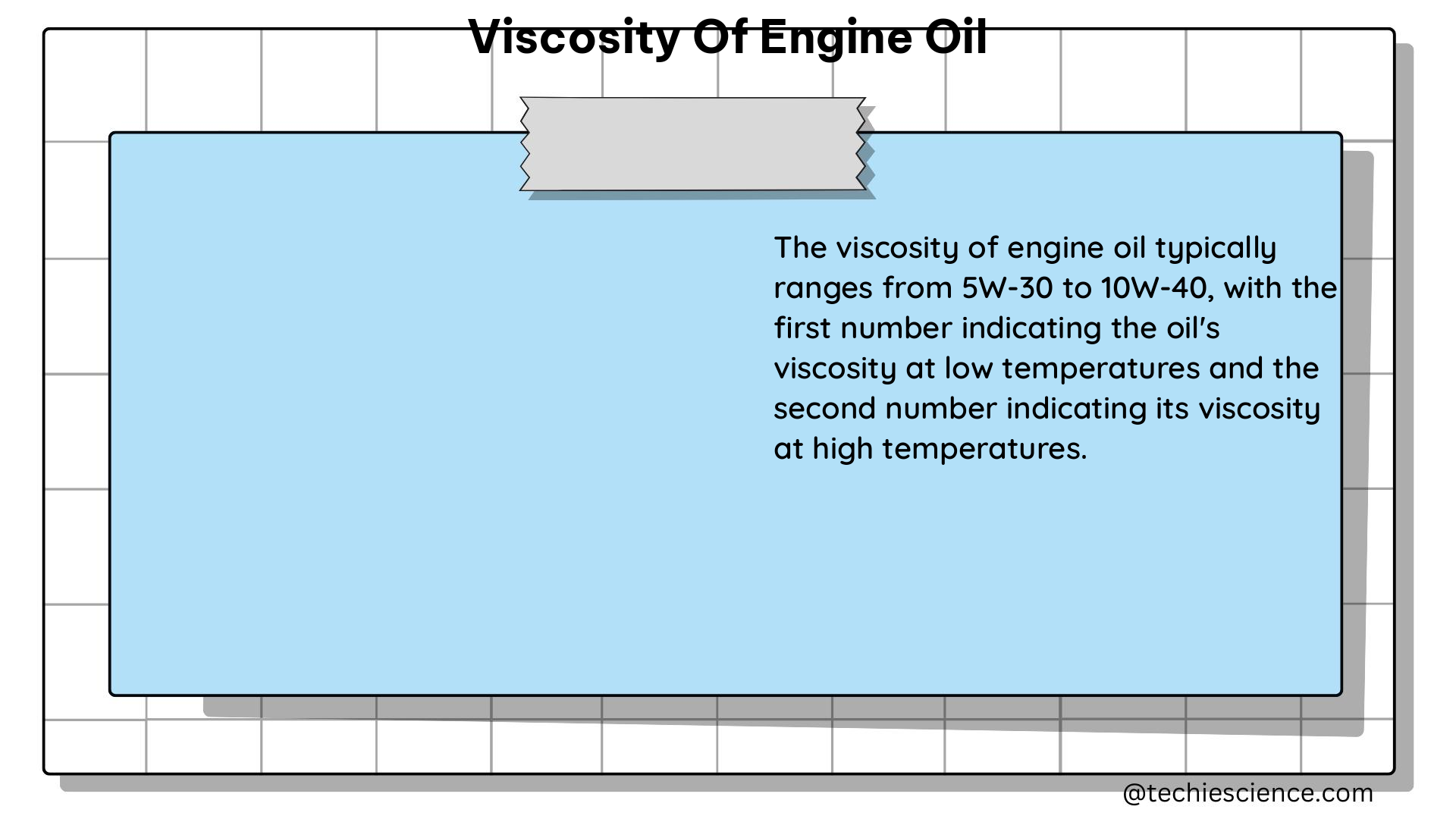
where P is the pressure and T is the temperature. The boiling point is a function of these two variables, but it does not depend on the amount of the substance.
Example 1: Boiling Point of Water
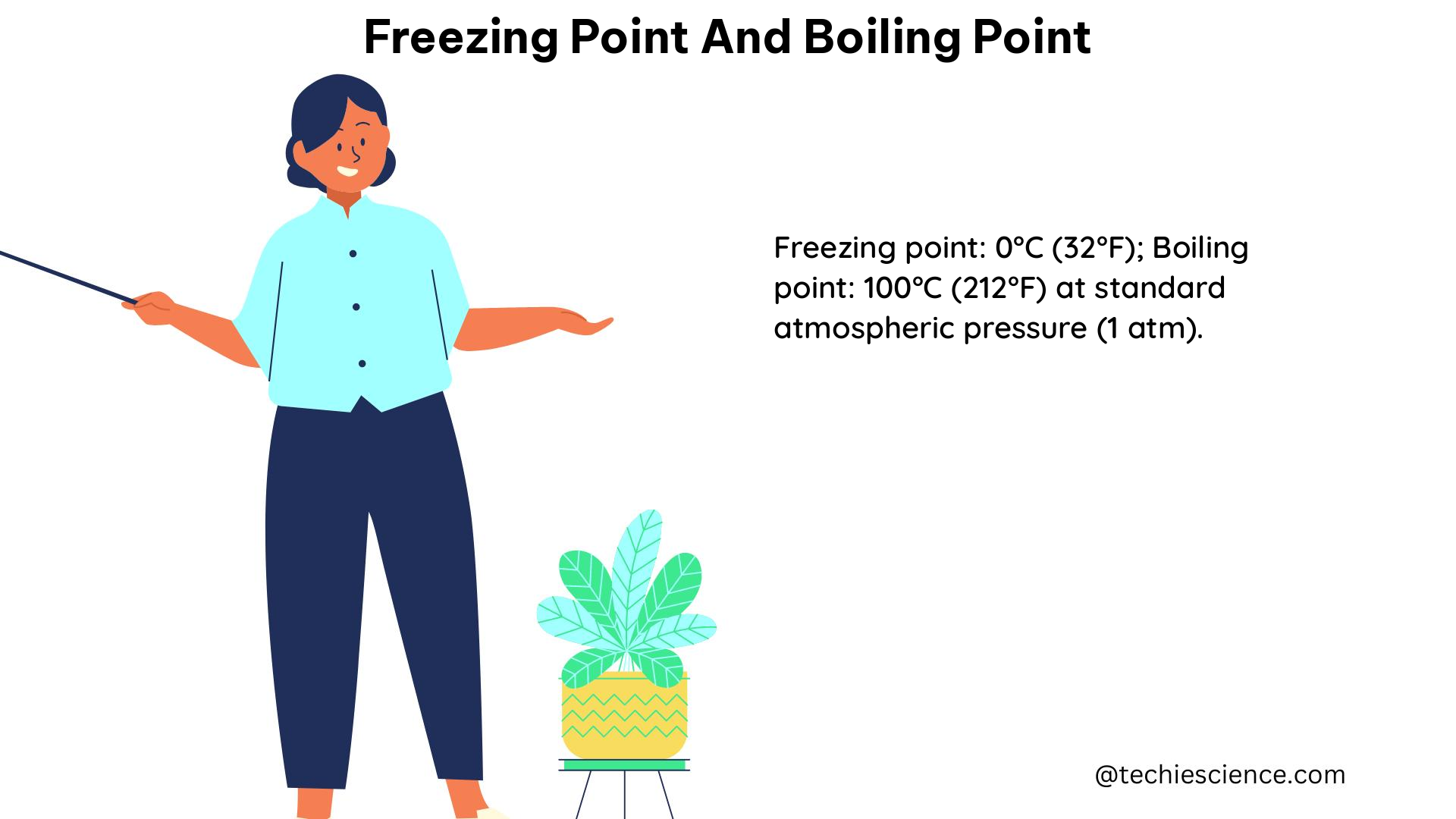
Consider the boiling point of water. At a pressure of 1 atmosphere (101.325 kPa), the boiling point of water is 100°C (212°F). This boiling point remains the same whether you have a small cup of water or a large container of water, as long as the pressure and temperature conditions are the same.
Example 2: Boiling Point of Ethanol
Similarly, the boiling point of ethanol (C₂H₅OH) at 1 atmosphere is 78.3°C (172.9°F). This boiling point is a characteristic of the ethanol molecule and does not depend on the amount of ethanol present in the system.
Numerical Problem
To further illustrate the concept, let’s consider a numerical problem:
Problem: Two containers, A and B, are filled with the same substance. Container A has a volume of 500 mL, while container B has a volume of 1000 mL. If the boiling point of the substance in both containers is measured to be 80°C, what can you conclude about the boiling point as an intensive property?
Solution: The fact that the boiling point of the substance is the same (80°C) in both containers, despite the difference in volume, demonstrates that the boiling point is an intensive property. The boiling point does not depend on the amount of the substance present, but rather on the specific characteristics of the substance and the environmental conditions (pressure and temperature).
Factors Affecting Boiling Point
While the boiling point is an intensive property, it can be influenced by various factors, such as:
- Pressure: The boiling point of a substance is directly related to the surrounding pressure. As the pressure increases, the boiling point also increases, and vice versa.
- Impurities: The presence of impurities in a substance can affect its boiling point. Impurities can either raise or lower the boiling point, depending on their nature and concentration.
- Molecular Structure: The molecular structure of a substance can also influence its boiling point. Substances with stronger intermolecular forces, such as hydrogen bonding, generally have higher boiling points.
Applications of Boiling Point as an Intensive Property
The understanding of boiling point as an intensive property has numerous applications in various fields, including:
- Chemical Identification: The boiling point of a substance is a characteristic property that can be used to identify and differentiate between different chemicals.
- Purification Processes: Boiling point is a crucial factor in distillation and other purification processes, where the separation of components is based on their different boiling points.
- Engineering and Technology: Boiling point is an important consideration in the design and operation of various systems, such as steam engines, refrigeration systems, and heat exchangers.
- Environmental Studies: Boiling point data is used in the analysis of environmental samples, such as the detection of volatile organic compounds (VOCs) in air or water.
Conclusion
In summary, the boiling point of a substance is an intensive property, which means it does not depend on the amount of the substance present. This property is essential in various fields of science and engineering, as it helps in the identification, characterization, and manipulation of substances. Understanding the intricacies of boiling point and its relationship with intensive and extensive properties is crucial for a comprehensive understanding of the physical and chemical behavior of materials.
Reference:
- Atkins, P., & de Paula, J. (2014). Atkins’ Physical Chemistry (10th ed.). Oxford University Press.
- Silbey, R. J., Alberty, R. A., & Bawendi, M. G. (2005). Physical Chemistry (4th ed.). Wiley.
- Chang, R., & Goldsby, K. A. (2013). Chemistry (11th ed.). McGraw-Hill Education.
- Zumdahl, S. S., & Zumdahl, S. A. (2013). Chemistry (9th ed.). Cengage Learning.
- Intensive and Extensive Properties – Wikipedia. (n.d.). Retrieved from https://en.wikipedia.org/wiki/Intensive_and_extensive_properties