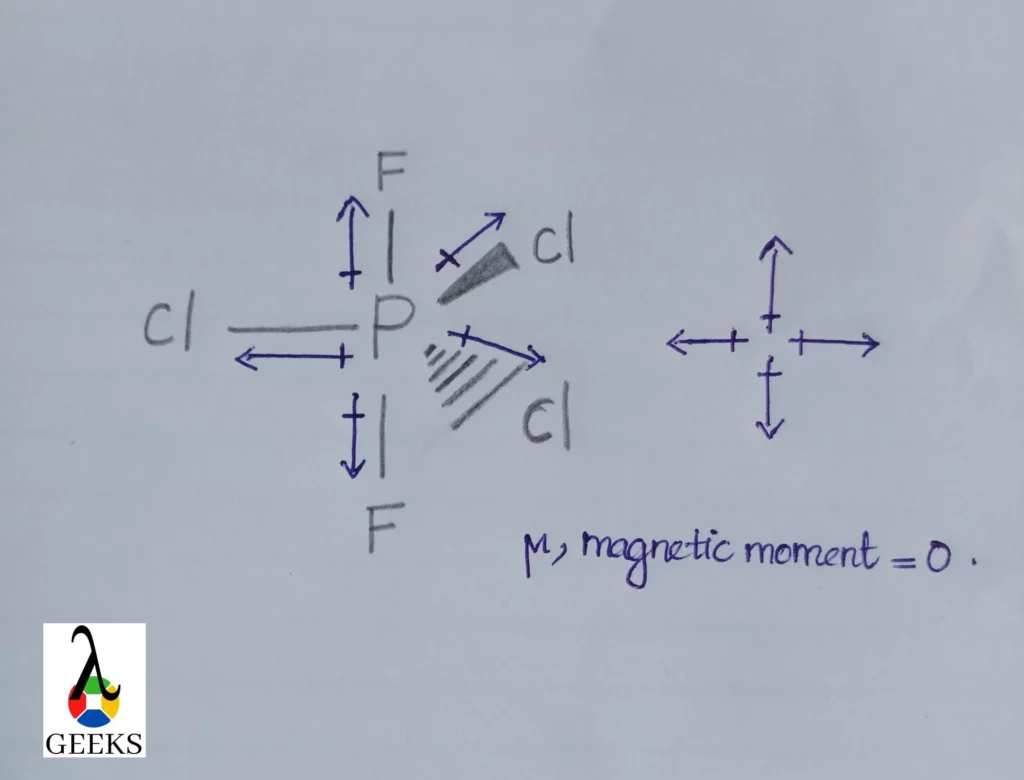
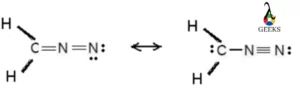
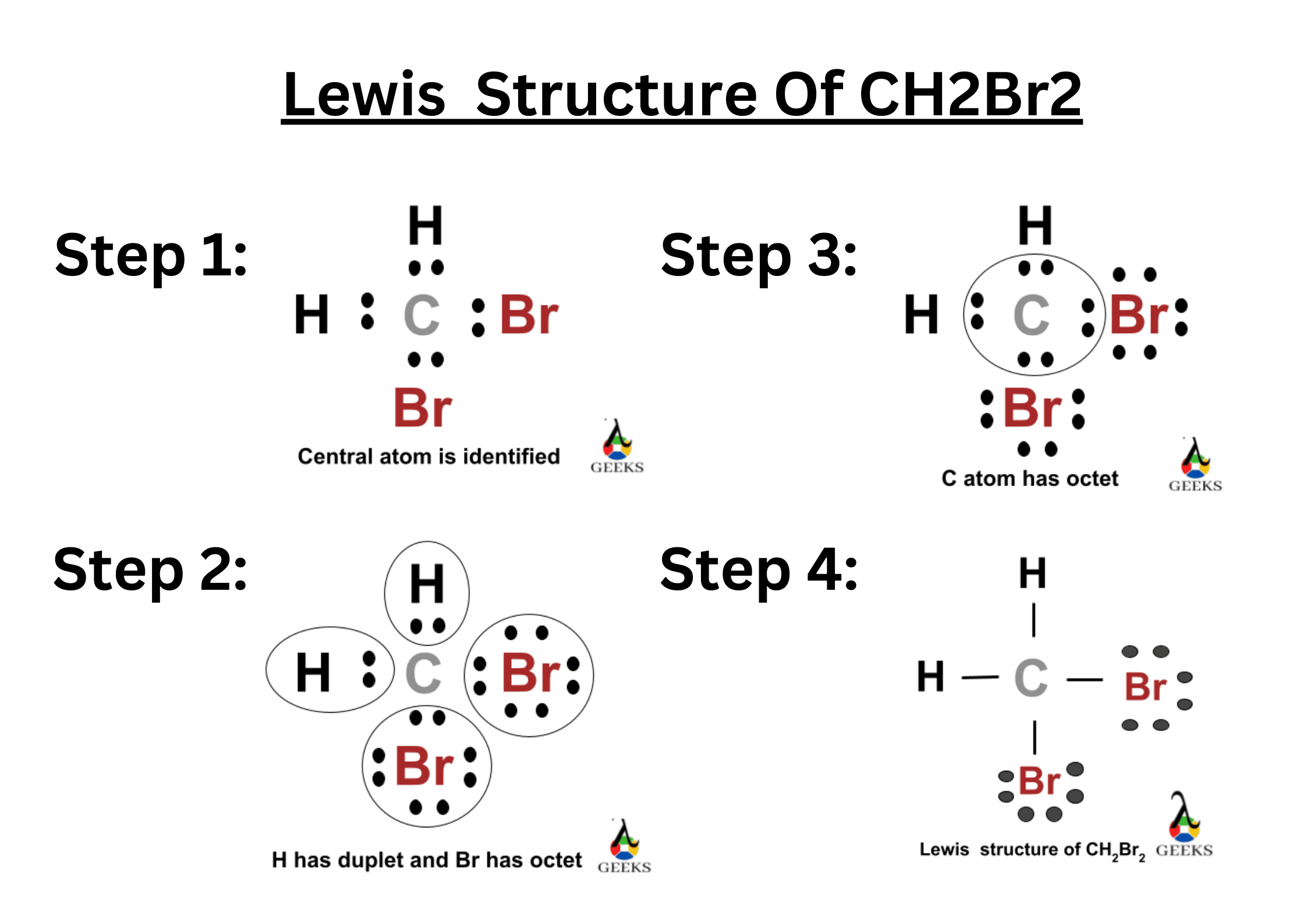
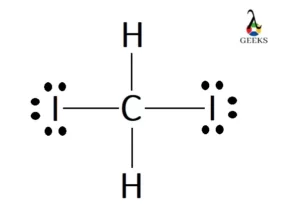
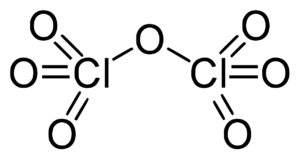
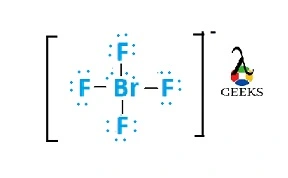
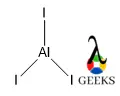
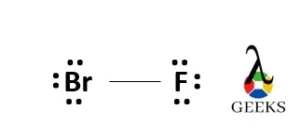N2F4 Lewis Structure & Characteristics (15 Helpful Facts)
Lewis Structure of H2PO2- The Lewis structure is a representation of the valence electrons in a molecule, showing how they are arranged around the atoms. In this section, we will explore the steps involved in drawing the Lewis structure of H2PO2-. Valence Electron Calculation Before we begin drawing the Lewis structure, let’s first determine the … Read more