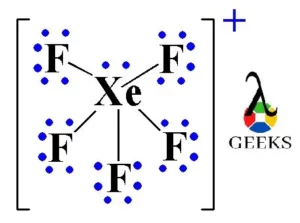
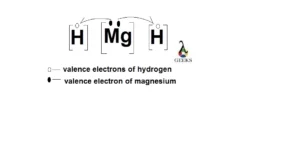
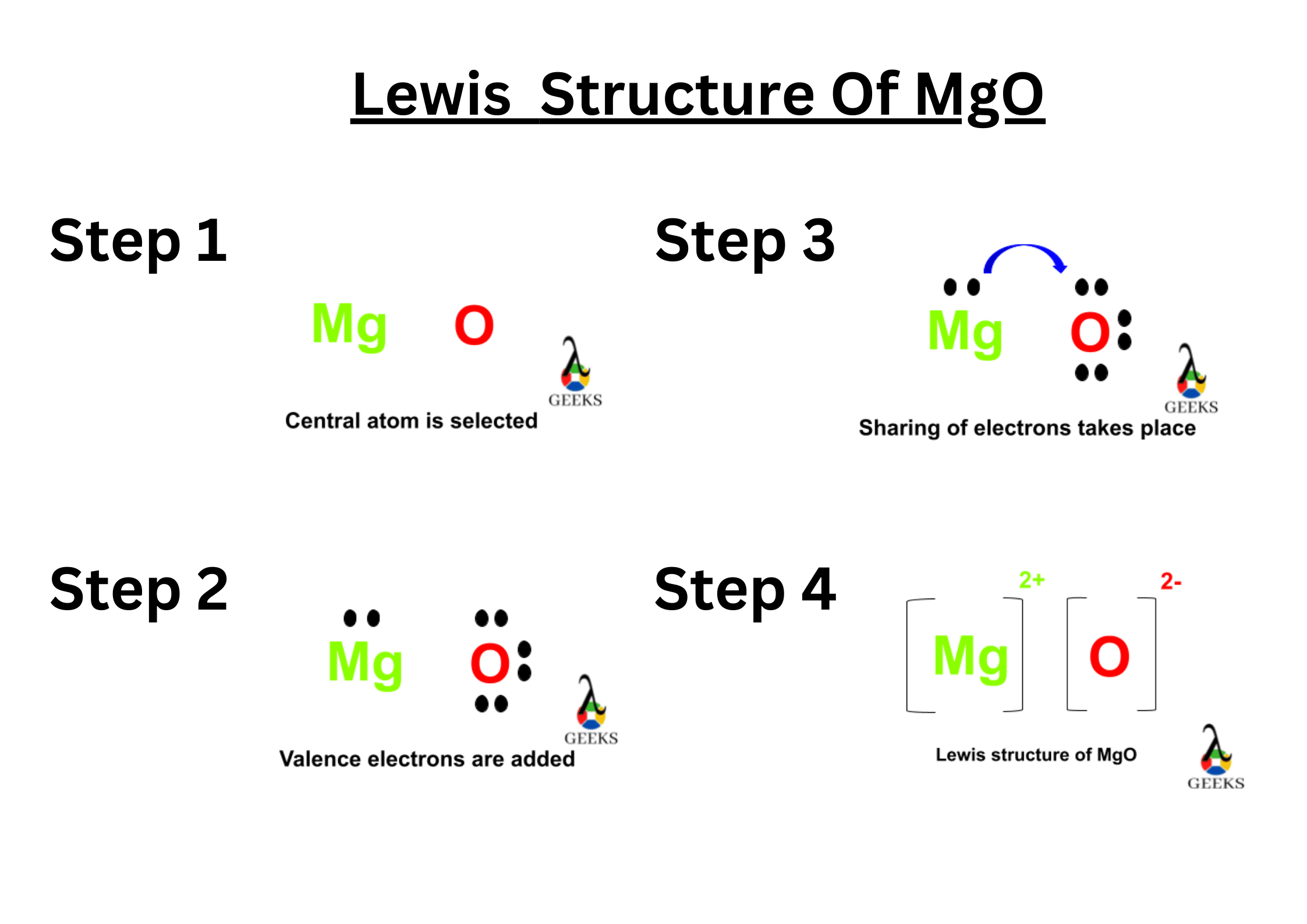
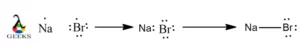
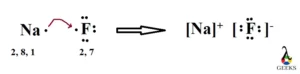
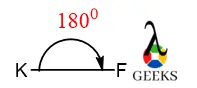
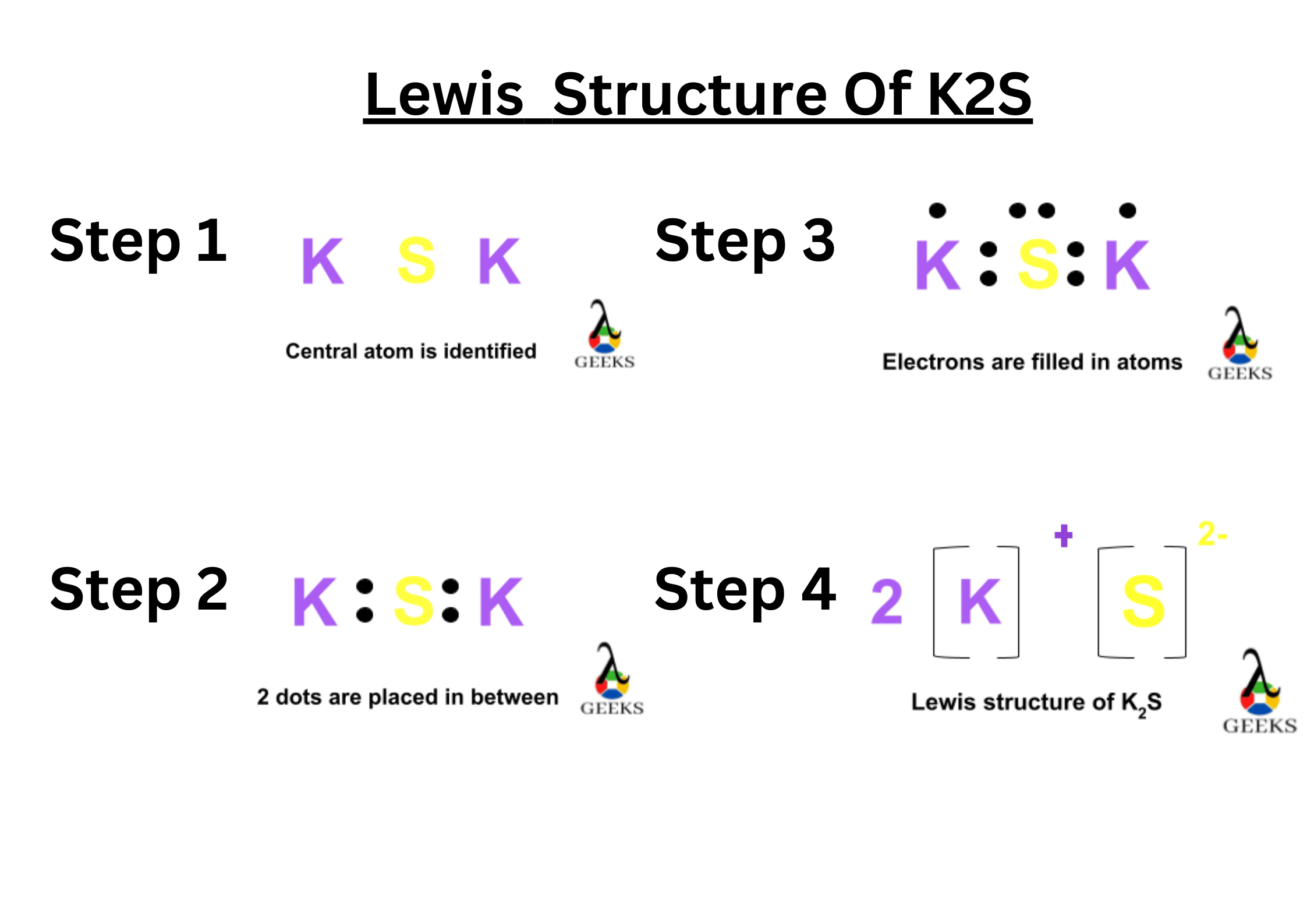
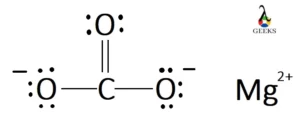XeF5+ Lewis Structure & Characteristics: 13 Complete Facts
XeF5 Lewis Structure is a topic that deals with the arrangement of atoms and electrons in a molecule of xenon pentafluoride. Lewis structures are diagrams that show the bonding between atoms in a molecule and the lone pairs of electrons that may exist. In the case of XeF5, it is important to understand the structure … Read more