The boiling point of a substance is the temperature at which its vapor pressure equals the external pressure, typically 1 atm or 101.3 kPa. The heat of vaporization, also known as the enthalpy of vaporization, is the amount of heat energy required to convert a unit mass or mole of a substance from its liquid phase to its gas phase at a constant temperature. This comprehensive guide will delve into the intricacies of these two fundamental concepts in physics and chemistry.
Understanding Boiling Point
The boiling point of a substance is the temperature at which the vapor pressure of the liquid equals the pressure surrounding the liquid, and bubbles of vapor form inside the liquid. This occurs when the vapor pressure of the liquid is equal to the pressure exerted on the liquid by the surrounding atmosphere.
The boiling point of a substance is affected by several factors, including:
-
Atmospheric Pressure: The boiling point of a substance is inversely proportional to the external pressure. As the pressure decreases, the boiling point also decreases. This is why water boils at a lower temperature at higher altitudes.
-
Intermolecular Forces: The strength of the intermolecular forces between the molecules of the substance affects the boiling point. Substances with stronger intermolecular forces, such as hydrogen bonding, generally have higher boiling points.
-
Molecular Mass: Heavier molecules generally have higher boiling points than lighter molecules, all other factors being equal.
-
Solute Concentration: The presence of solutes in a solution can affect the boiling point. The boiling point of a solution is typically higher than the boiling point of the pure solvent, a phenomenon known as boiling point elevation.
To calculate the boiling point of a substance, you can use the following formula:
$T_b = T_0 + K_b \cdot m$
Where:
– $T_b$ is the boiling point of the solution
– $T_0$ is the boiling point of the pure solvent
– $K_b$ is the boiling point elevation constant, which is specific to the solvent
– $m$ is the molality of the solution (moles of solute per kilogram of solvent)
Heat of Vaporization
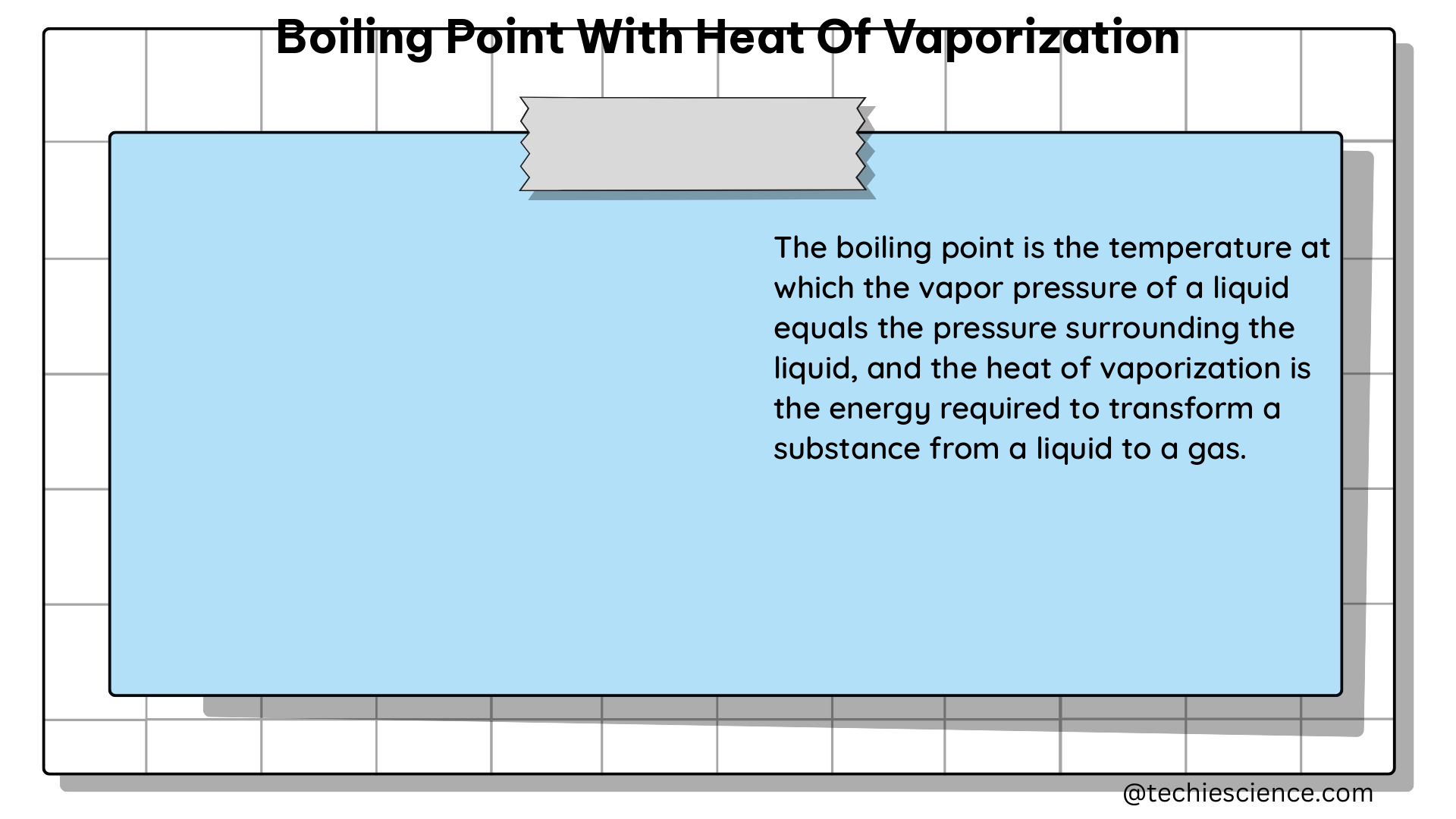
The heat of vaporization, also known as the enthalpy of vaporization, is the amount of energy required to convert a unit mass or mole of a substance from its liquid phase to its gas phase at a constant temperature and pressure. This energy is required to overcome the intermolecular forces that hold the liquid molecules together.
The heat of vaporization can be calculated using the following formula:
$\Delta H_v = \frac{RT_b^2}{T_b – T_a}$
Where:
– $\Delta H_v$ is the heat of vaporization
– $R$ is the universal gas constant (8.314 J/mol·K)
– $T_b$ is the boiling point of the substance in Kelvin
– $T_a$ is the temperature at which the heat of vaporization is being calculated
The heat of vaporization is an important property in many applications, such as:
-
Refrigeration: The heat of vaporization is a key factor in the design and efficiency of refrigeration systems, as it determines the amount of energy required to evaporate the refrigerant.
-
Boiling and Evaporation: The heat of vaporization affects the rate of boiling and evaporation, which is important in processes like distillation, drying, and steam generation.
-
Atmospheric Science: The heat of vaporization plays a crucial role in the water cycle and atmospheric processes, such as the formation of clouds and precipitation.
-
Chemical Processes: The heat of vaporization is a critical parameter in various chemical processes, including the design of chemical reactors and the optimization of energy-intensive operations like distillation and drying.
Clausius-Clapeyron Equation
The relationship between the boiling point and the heat of vaporization is described by the Clausius-Clapeyron equation, which can be used to estimate the vapor pressure of a substance at a different temperature if its vapor pressure and heat of vaporization are known at a reference temperature. The equation is as follows:
$\ln\left(\frac{P_2}{P_1}\right) = \left(-\frac{\Delta H_v}{R}\right)\left(\frac{1}{T_2} – \frac{1}{T_1}\right)$
Where:
– $P_1$ and $P_2$ are the vapor pressures at the reference temperature $T_1$ and the unknown temperature $T_2$, respectively
– $\Delta H_v$ is the heat of vaporization
– $R$ is the gas constant
– $T_1$ and $T_2$ are the absolute temperatures in Kelvin
This equation can be rearranged to solve for the unknown vapor pressure $P_2$ or the unknown temperature $T_2$.
Example Calculation
Let’s consider the example provided in the original question:
If we know that the vapor pressure of water at 100°C is 1 atm or 101.3 kPa, and its heat of vaporization is 40.67 kJ/mol, we can use the Clausius-Clapeyron equation to estimate its vapor pressure at 110°C or 383.15 K.
Substituting the values into the equation:
$\ln\left(\frac{P_2}{101.3}\right) = \left(-\frac{40670}{8.314}\right)\left(\frac{1}{383.15} – \frac{1}{373.15}\right)$
Solving for $P_2$:
$P_2 = 101.3 \times \exp\left(\left(-\frac{40670}{8.314}\right)\left(\frac{1}{383.15} – \frac{1}{373.15}\right)\right)$
$P_2 \approx 130.3 \text{ kPa}$
Therefore, the vapor pressure of water at 110°C is approximately 130.3 kPa.
Practical Applications and Examples
The concepts of boiling point and heat of vaporization have numerous practical applications in various fields, including:
-
Chemical Engineering: In the design of distillation columns, evaporators, and other separation processes, the boiling point and heat of vaporization of the components are crucial parameters.
-
Meteorology and Atmospheric Science: The heat of vaporization plays a vital role in the water cycle and the formation of clouds and precipitation.
-
Refrigeration and Air Conditioning: The heat of vaporization is a key factor in the design and efficiency of refrigeration systems, as it determines the amount of energy required to evaporate the refrigerant.
-
Pharmaceutical and Food Industries: The boiling point and heat of vaporization are important in processes like drying, lyophilization, and spray drying, which are commonly used in the production of pharmaceuticals and food products.
-
Materials Science: The boiling point and heat of vaporization are relevant in the synthesis and processing of materials, such as the deposition of thin films and the growth of crystals.
-
Energy Production: The heat of vaporization is a crucial parameter in the design and operation of steam power plants, where the conversion of water to steam is a key step in the energy generation process.
Conclusion
The boiling point and heat of vaporization are fundamental concepts in physics and chemistry that have far-reaching applications in various fields. Understanding these concepts and their underlying principles is essential for scientists, engineers, and researchers working in diverse areas, from chemical processing to atmospheric science and beyond. This comprehensive guide has provided a detailed exploration of these topics, including the factors that influence boiling point, the calculation of heat of vaporization, and the application of the Clausius-Clapeyron equation. By mastering these concepts, you can unlock a deeper understanding of the physical world and contribute to the advancement of scientific and technological innovations.