Zinc Electron Configuration:7 Easy Step-by-Step Guide
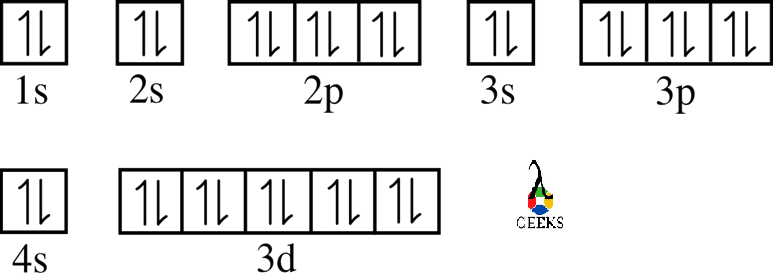
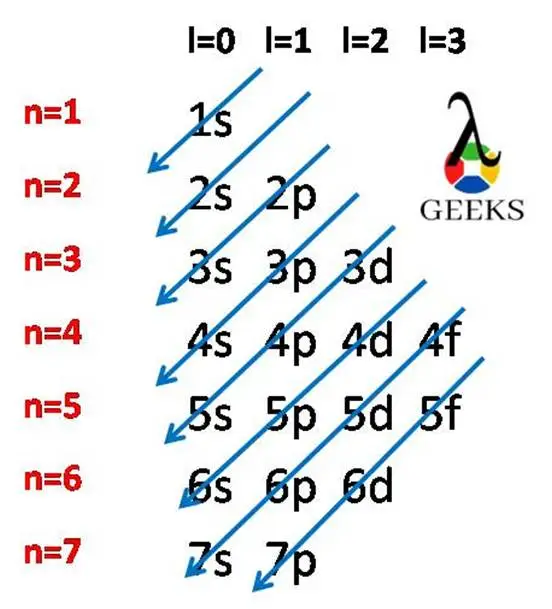
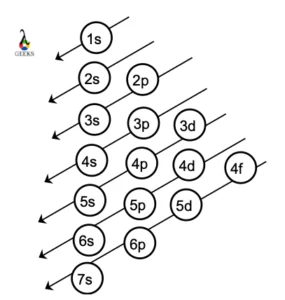
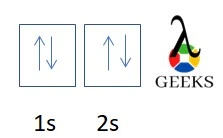
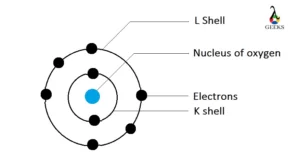
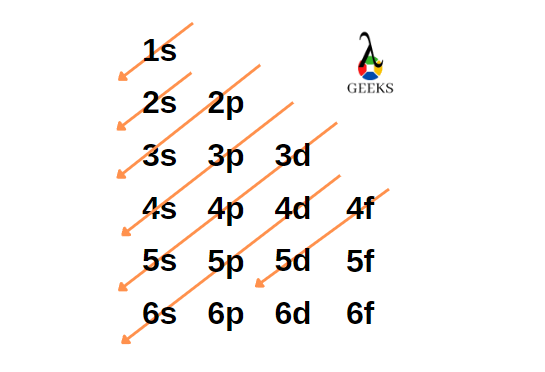
Zinc represents the symbol Zn in the periodic table. Let us discuss about electronic configuration of zinc in this article. The electronic configuration of zinc is 1s2 2s2 2p6 3s2 3p6 4s2 3d10. Electrons are distributed among the shells K, L, M, and N. Zn is the 12 th group element and one of the … Read more