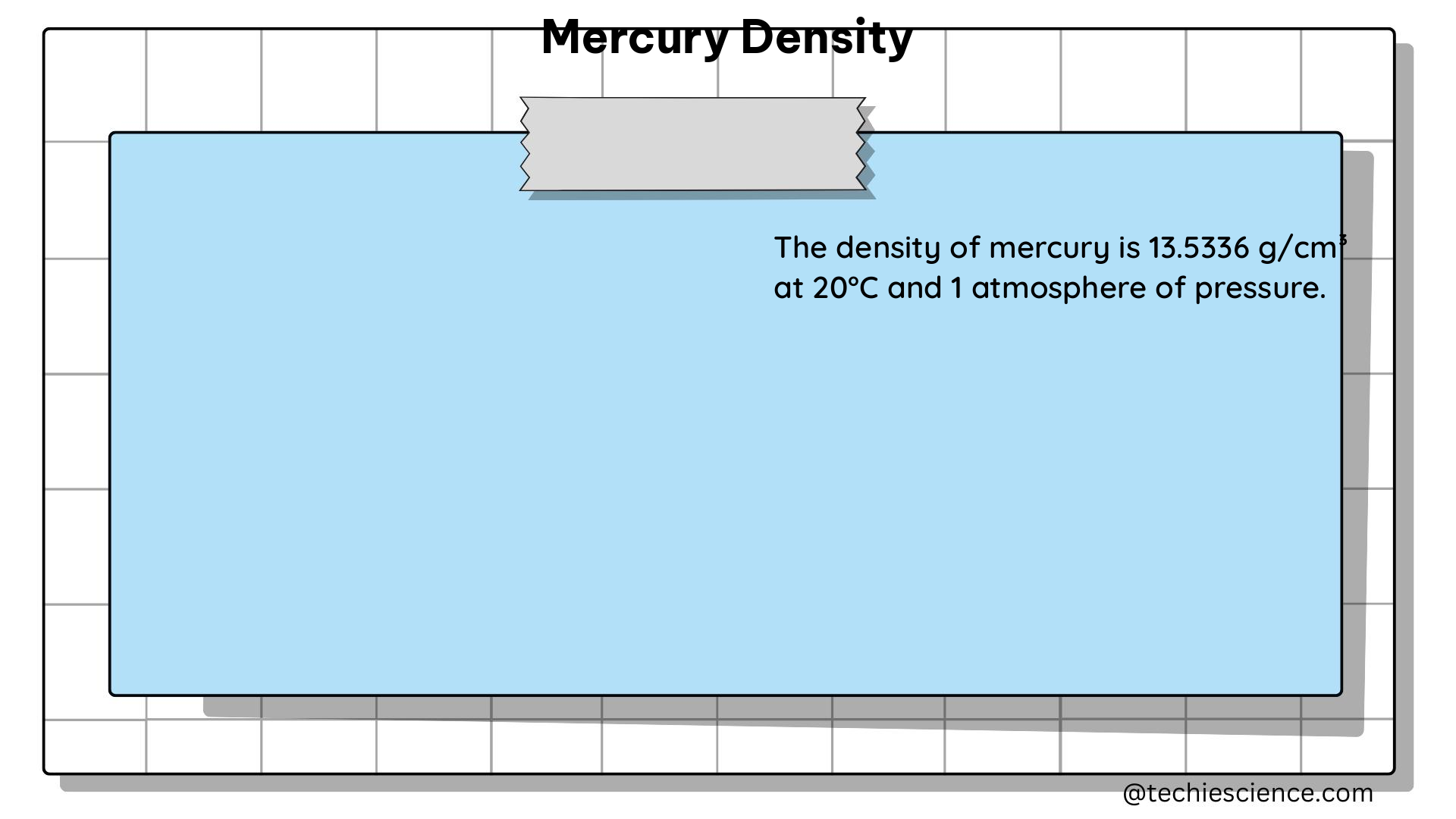
Density is a fundamental property of matter that is widely used in various fields of science and engineering. It is an intensive property, meaning that it does not depend on the amount of material being measured. This is a crucial concept in physics and chemistry, as it allows for the comparison and analysis of different substances and materials. In this comprehensive blog post, we will delve into the intricacies of density as an intensive property, exploring the underlying principles, mathematical formulations, and practical applications.
Understanding Density as an Intensive Property
Density is defined as the ratio of the mass of an object or substance to its volume. Mathematically, it can be expressed as:
Density = Mass / Volume
The key aspect that makes density an intensive property is that the ratio of mass to volume remains constant, regardless of the amount of material being measured. This is because both mass and volume are extensive properties, meaning they depend on the amount of material. However, when the ratio is taken, the dependence on the amount of material cancels out, resulting in an intensive property.
To illustrate this concept, let’s consider two samples of the same substance, one with twice the mass and volume of the other. The density of both samples will be the same, as shown in the following calculations:
Sample 1:
Mass = 100 g
Volume = 100 mL
Density = 100 g / 100 mL = 1 g/mL
Sample 2:
Mass = 200 g
Volume = 200 mL
Density = 200 g / 200 mL = 1 g/mL
As you can see, the density of both samples is 1 g/mL, despite the difference in their mass and volume. This demonstrates the key characteristic of an intensive property: it remains constant regardless of the amount of material being measured.
Extensive and Intensive Properties

To better understand the concept of density as an intensive property, it is important to distinguish between extensive and intensive properties.
Extensive Properties:
– Depend on the amount of material being measured
– Examples: mass, volume, length, area, and energy
Intensive Properties:
– Do not depend on the amount of material being measured
– Examples: density, temperature, pressure, and concentration
The distinction between these two types of properties is crucial in various areas of physics and chemistry, as it allows for the comparison and analysis of different substances and materials.
Calculating Density
The calculation of density involves the measurement of two extensive properties: mass and volume. The formula for density is:
Density = Mass / Volume
This formula can be used to calculate the density of any substance or object, provided that the mass and volume are known.
It is important to note that the units of density can vary depending on the system of measurement being used. In the International System of Units (SI), the standard unit of density is kilograms per cubic meter (kg/m³). However, other units, such as grams per milliliter (g/mL) or pounds per cubic foot (lb/ft³), are also commonly used.
Factors Affecting Density
The density of a substance or object can be influenced by several factors, including:
-
Composition: The chemical composition of a material can affect its density. For example, the density of water is different from the density of iron or gold.
-
Temperature: The density of a substance can change with temperature. As a general rule, the density of most substances decreases as the temperature increases, due to the expansion of the material.
-
Pressure: The density of a substance can also be affected by pressure. Increasing the pressure on a material can cause it to become more compact, resulting in a higher density.
-
Phase: The phase of a substance (solid, liquid, or gas) can significantly impact its density. For instance, the density of water in its liquid form is different from its density in the solid (ice) or gaseous (steam) forms.
Understanding these factors is crucial in various applications, such as the design of materials, the analysis of fluid dynamics, and the study of phase changes in substances.
Practical Applications of Density as an Intensive Property
The fact that density is an intensive property has numerous practical applications in various fields, including:
-
Material Identification: Density can be used to identify and differentiate between different materials, as each substance has a unique density value.
-
Buoyancy and Flotation: Density is a key factor in determining the buoyancy of objects in fluids, which is essential for understanding phenomena such as floating and sinking.
-
Fluid Mechanics: Density is a crucial parameter in the study of fluid dynamics, as it is used in the calculation of pressure, flow rate, and other important fluid properties.
-
Geological and Astronomical Applications: Density is widely used in the fields of geology and astronomy to study the composition and structure of planets, stars, and other celestial bodies.
-
Engineering and Design: Density is an important consideration in the design and construction of various structures, vehicles, and devices, as it affects factors such as weight, load-bearing capacity, and energy efficiency.
-
Chemical Analysis: Density measurements are often used in analytical chemistry to determine the purity and composition of substances, as well as to monitor chemical reactions and processes.
These are just a few examples of the many practical applications of density as an intensive property in science, engineering, and everyday life.
Conclusion
In conclusion, density is an intensive property that does not depend on the amount of material being measured. This fundamental characteristic of density allows for the comparison and analysis of different substances and materials, making it a crucial concept in various fields of science and engineering. By understanding the underlying principles, mathematical formulations, and practical applications of density as an intensive property, we can gain deeper insights into the behavior and properties of the physical world around us.
References:
- “Density: An Intensive Property of Matter” from the Carolina Knowledge Center
- “1.3: Properties of Matter” from Chemistry LibreTexts
- “Intensive and extensive properties” from Wikipedia