Melting point and density are two fundamental physical properties of matter that are crucial for understanding the behavior and characteristics of various substances. These properties not only help in identifying and distinguishing different materials but also play a vital role in various scientific and engineering applications. In this comprehensive guide, we will delve into the intricacies of melting point and density, providing you with a deep understanding of these concepts and their practical applications.
Understanding Melting Point
Melting point is the temperature at which a solid substance transitions from a solid state to a liquid state at a given pressure, typically atmospheric pressure. This transition occurs when the intermolecular forces holding the solid structure together are overcome by the thermal energy of the system. The melting point is a characteristic property of a pure substance, meaning that it remains constant for a given substance under the same conditions.
Factors Affecting Melting Point
The melting point of a substance can be influenced by several factors, including:
-
Chemical Composition: The chemical structure and composition of a substance play a crucial role in determining its melting point. Substances with stronger intermolecular forces, such as ionic or covalent bonds, generally have higher melting points compared to those with weaker intermolecular forces, such as van der Waals forces.
-
Pressure: The melting point of a substance can be affected by changes in pressure. According to the Clausius-Clapeyron equation, an increase in pressure generally leads to an increase in the melting point, while a decrease in pressure results in a lower melting point.
-
Impurities: The presence of impurities in a substance can alter its melting point. Impurities can disrupt the regular crystal structure of the substance, leading to a change in the melting point.
Measuring Melting Point
The melting point of a substance can be measured using various techniques, including:
-
Capillary Tube Method: In this method, a small sample of the substance is placed in a thin glass capillary tube, and the tube is then immersed in a heating bath. The temperature at which the substance begins to melt is recorded as the melting point.
-
Differential Scanning Calorimetry (DSC): DSC is a more advanced technique that measures the difference in heat flow between a sample and a reference material as a function of temperature. This method can provide detailed information about the melting point and other thermal properties of a substance.
-
Melting Point Apparatus: A melting point apparatus is a simple and commonly used device that consists of a heating plate and a thermometer. The sample is placed on the heating plate, and the temperature at which the substance begins to melt is observed and recorded.
Understanding Density
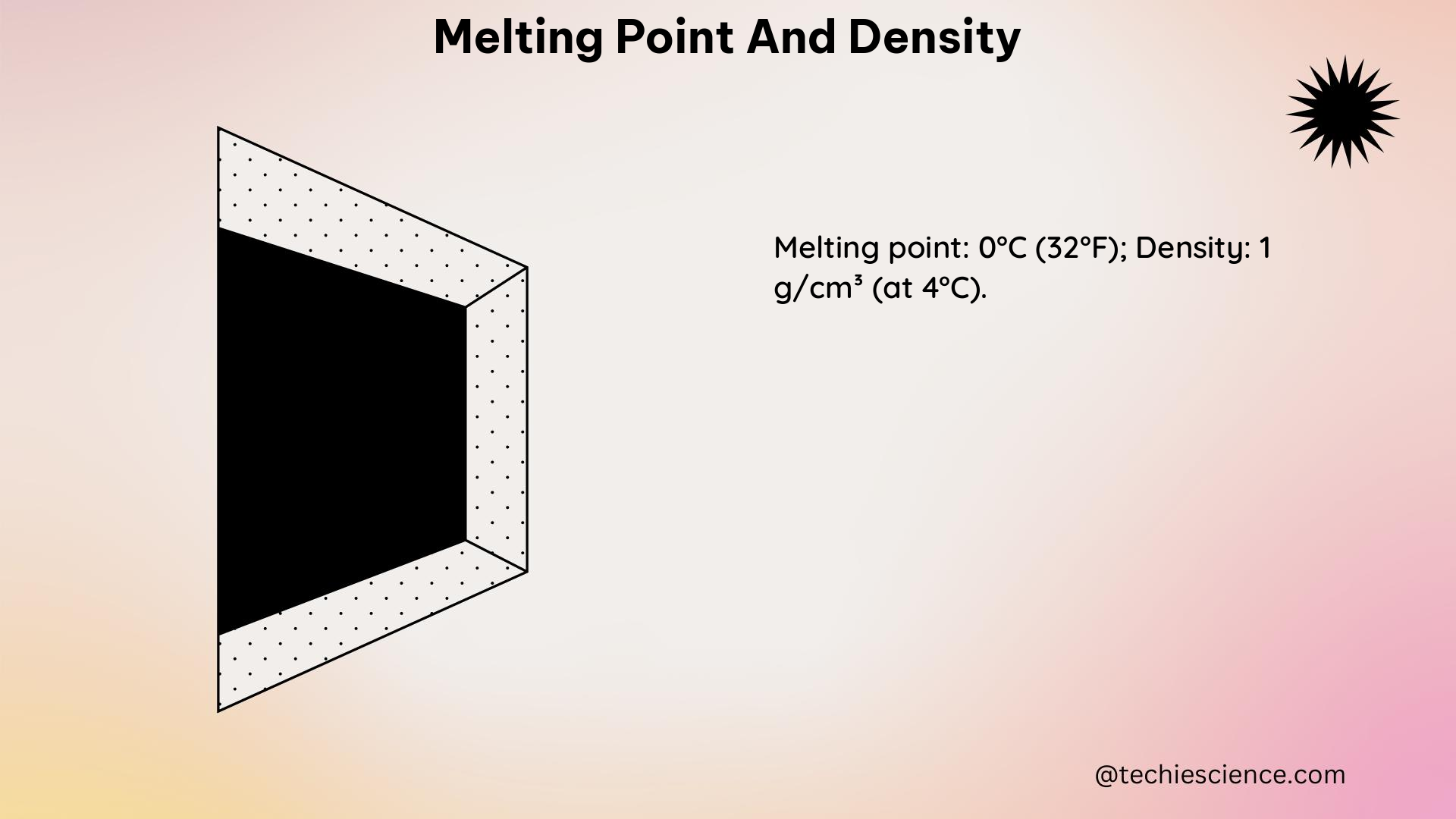
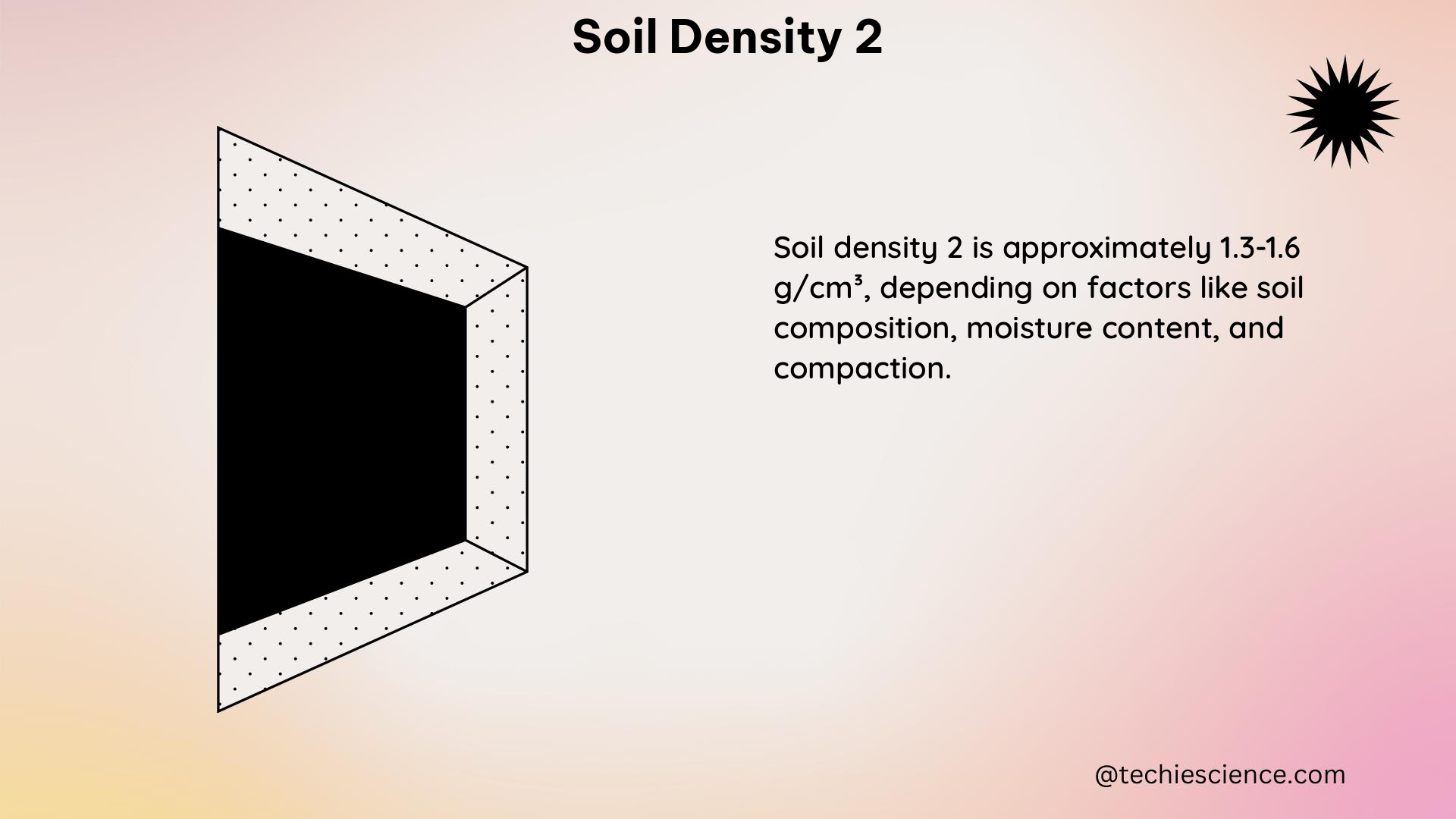
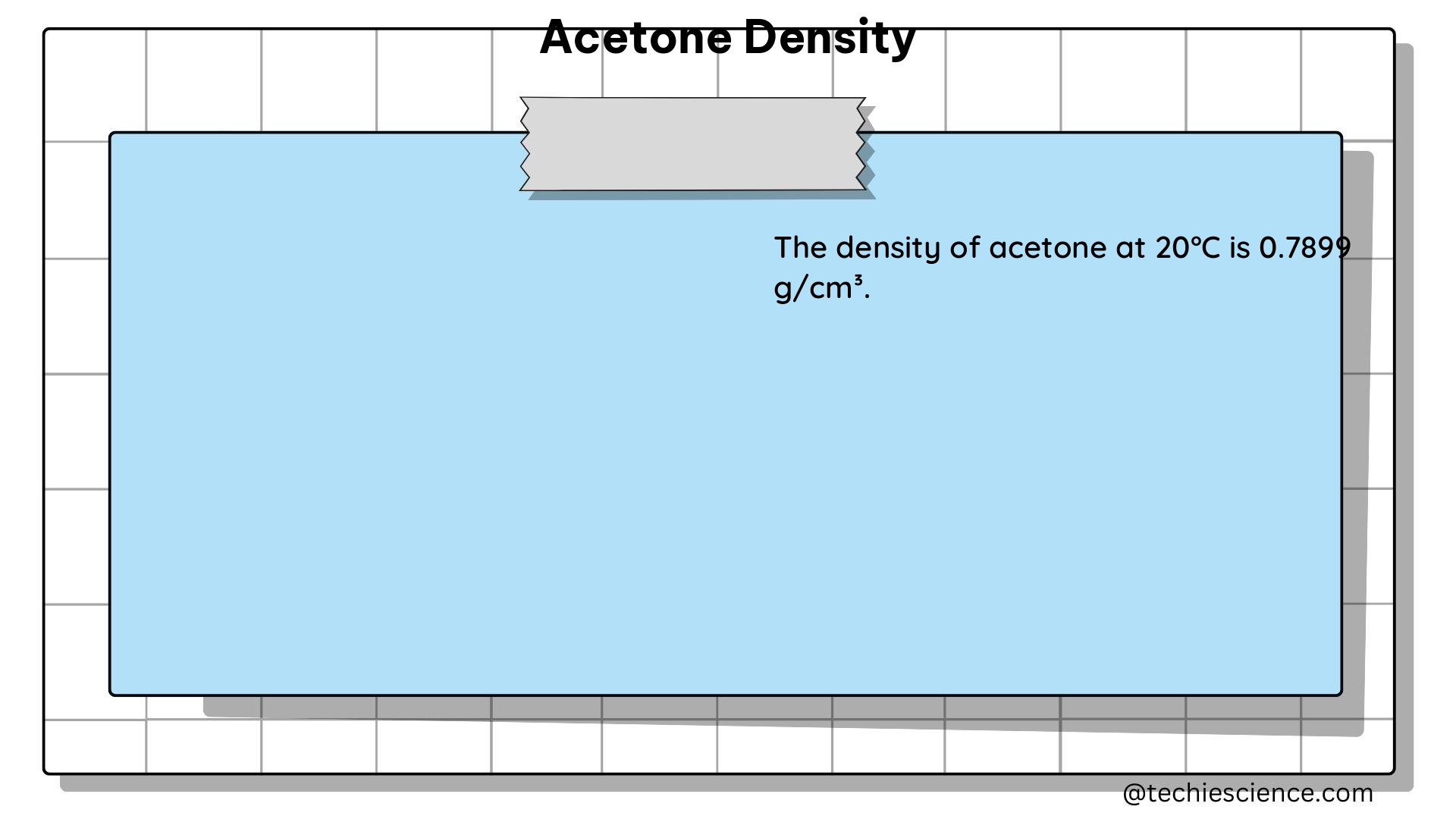
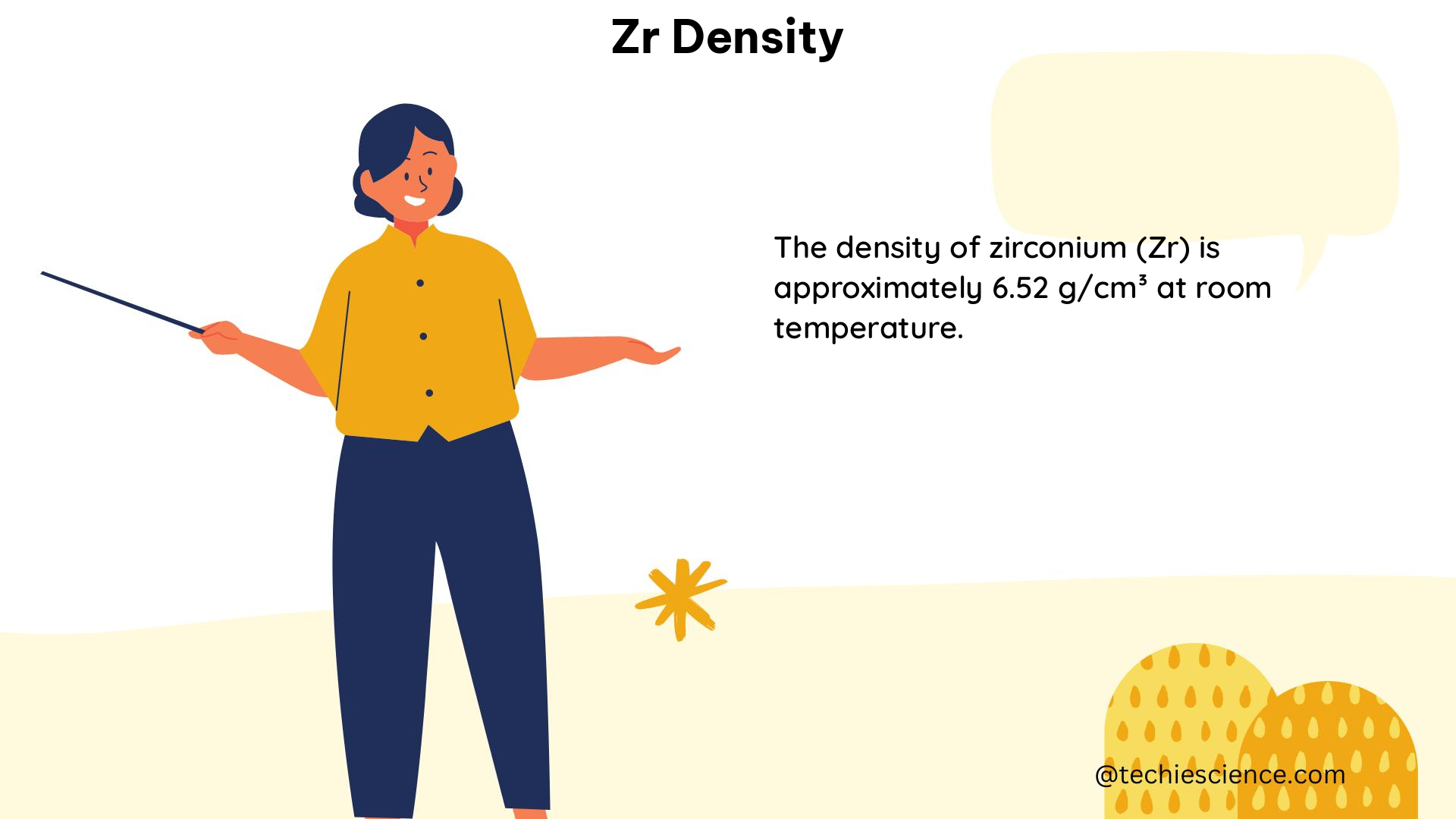
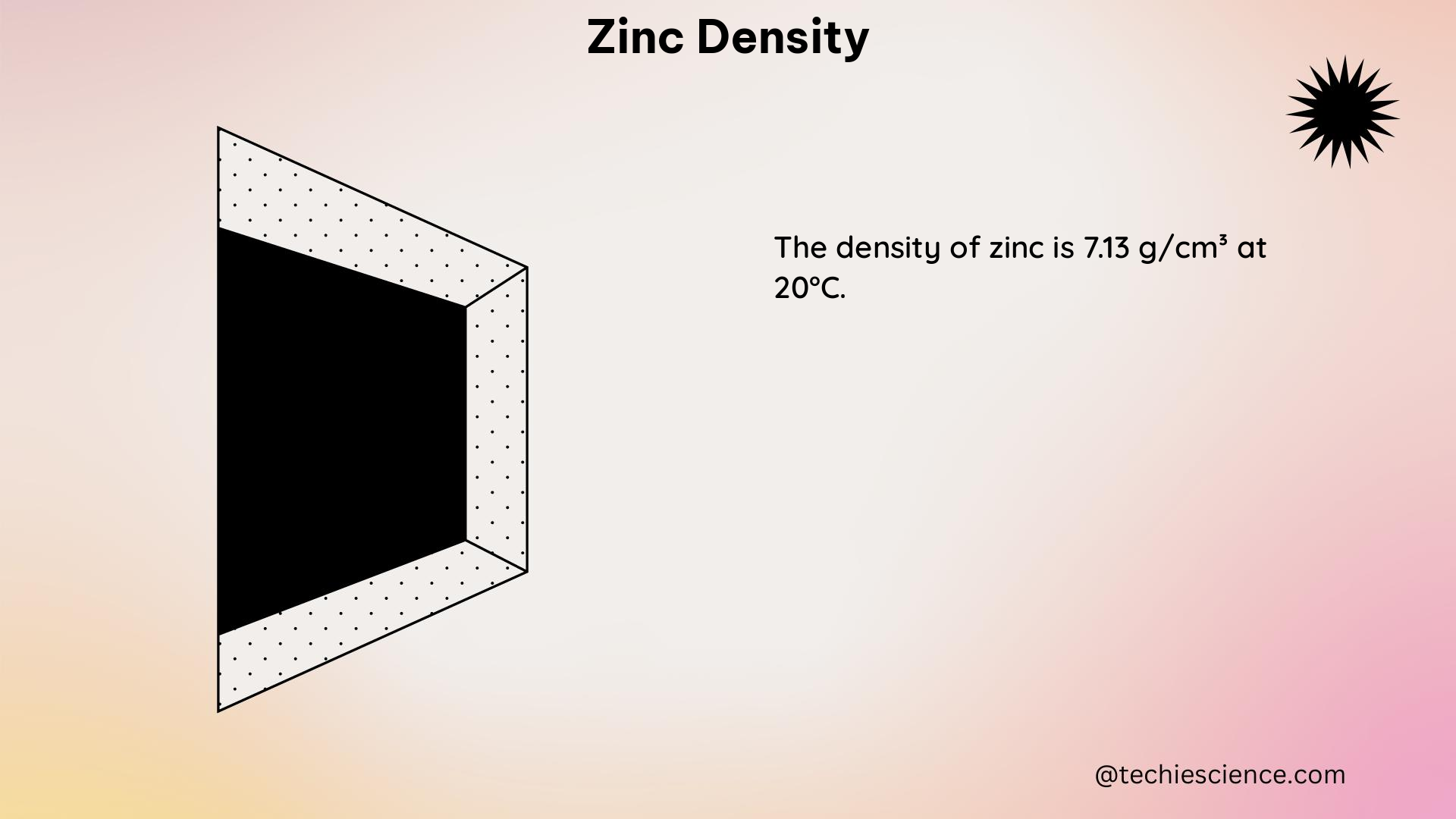
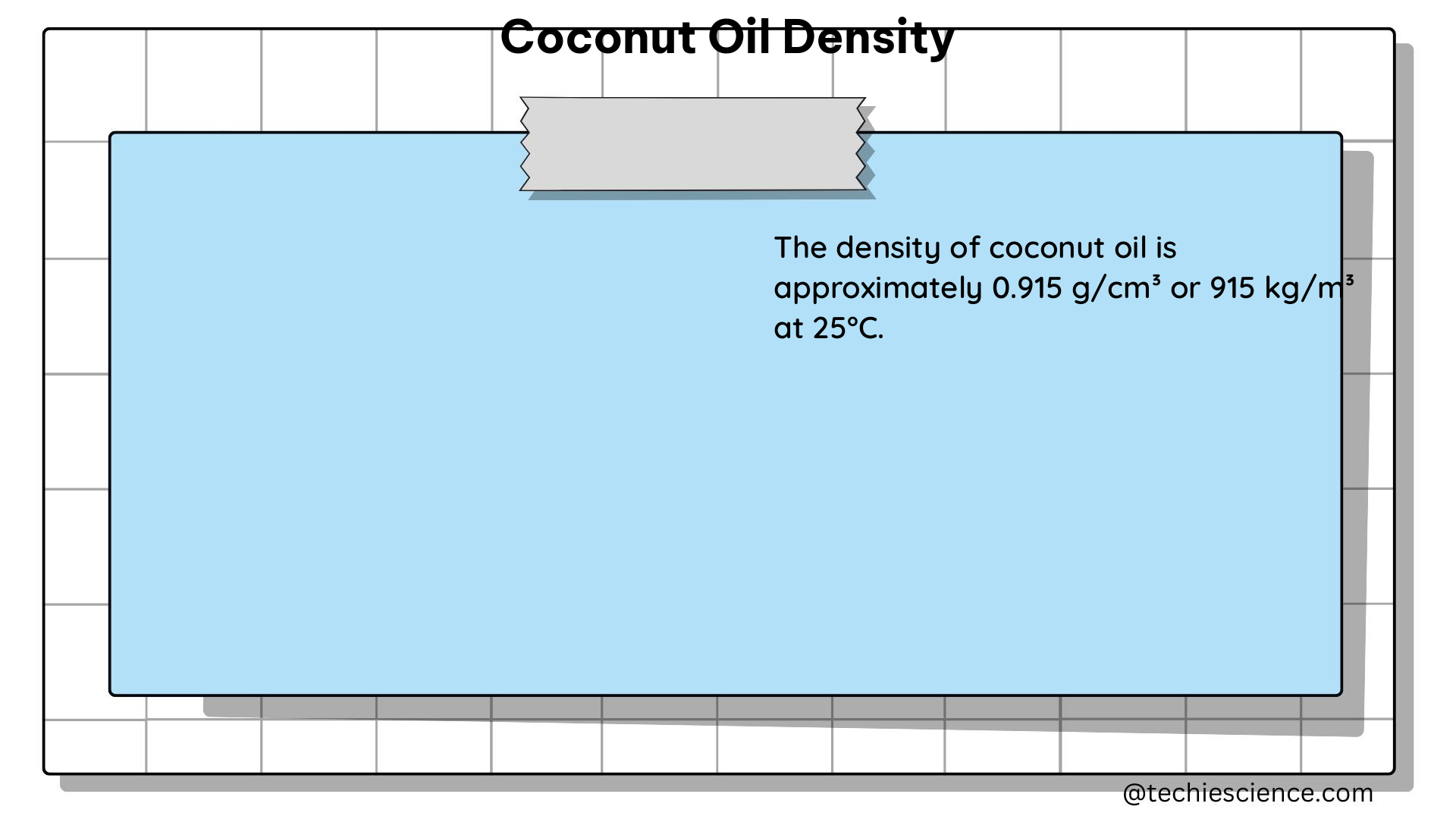
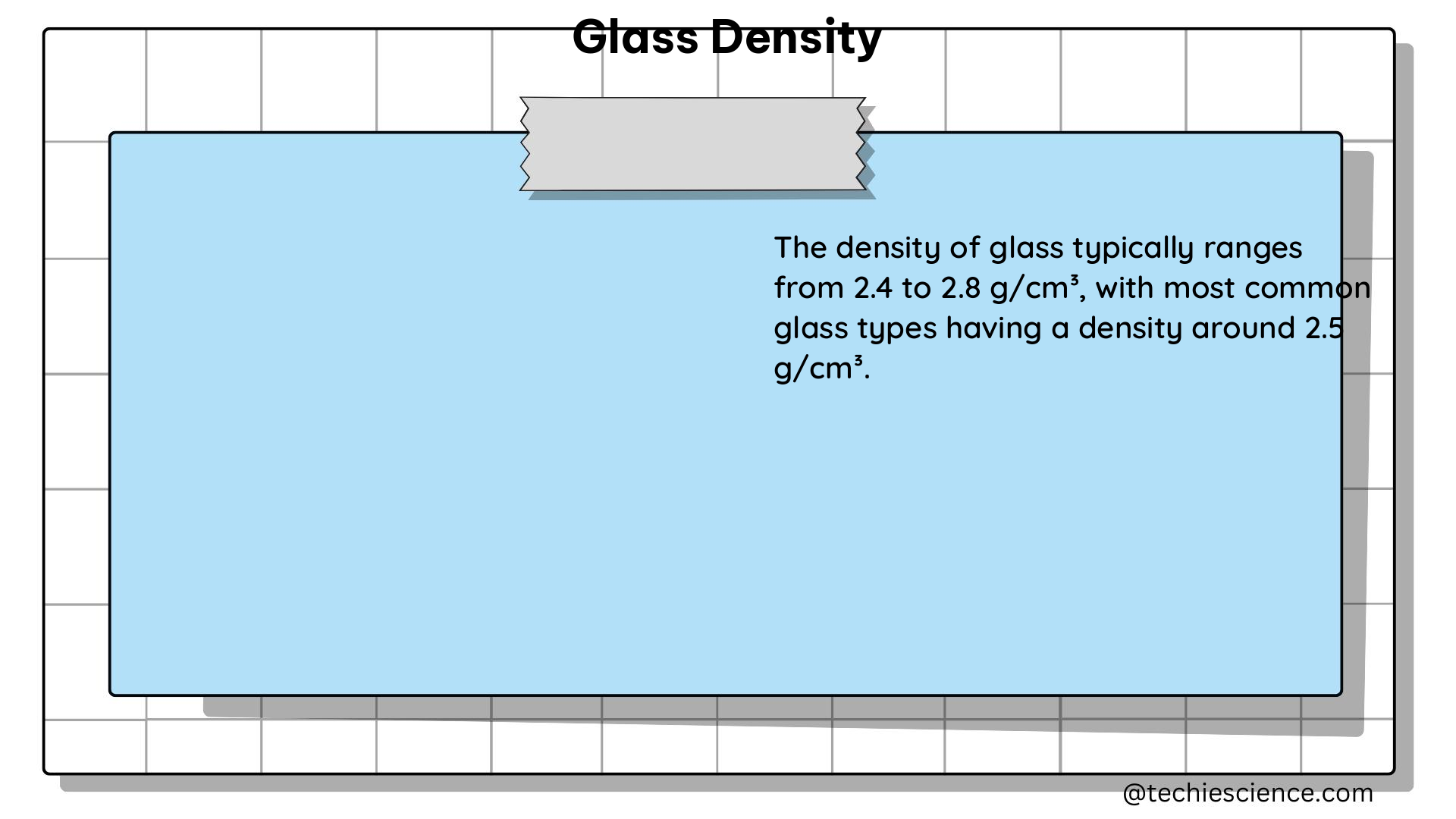
Density is a fundamental physical property that describes the mass of a substance per unit volume. It is an intensive property, meaning that it does not depend on the amount of the substance. Density is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
Factors Affecting Density
The density of a substance can be influenced by several factors, including:
-
Chemical Composition: The chemical structure and composition of a substance directly affect its density. Substances with higher atomic or molecular weights generally have higher densities.
-
Temperature: The density of a substance can change with temperature. As the temperature increases, the volume of a substance typically expands, leading to a decrease in density.
-
Pressure: Pressure can also affect the density of a substance. Increasing the pressure on a substance can cause it to become more compact, resulting in an increase in density.
Measuring Density
The density of a substance can be measured using various techniques, including:
-
Pycnometer Method: A pycnometer is a device that measures the volume of a given mass of a substance. By dividing the mass of the substance by its volume, the density can be calculated.
-
Buoyancy Method: This method involves measuring the buoyant force exerted on an object immersed in the substance. The density of the substance can then be calculated using the principle of Archimedes’ principle.
-
Displacement Method: In this method, the volume of a substance is determined by measuring the volume of water or another liquid displaced by a known mass of the substance.
Relationship between Melting Point and Density
The melting point and density of a substance are related through the Clausius-Clapeyron equation, which describes the relationship between the vapor pressure and temperature of a substance. This equation can be used to calculate the change in melting point with pressure, which is directly related to the density of the substance.
The Clausius-Clapeyron equation is given by:
(dP/dT) = (ΔHfus / T) / (ΔVfus)
Where:
– dP/dT is the change in pressure with respect to temperature
– ΔHfus is the latent heat of fusion
– T is the absolute temperature
– ΔVfus is the change in volume during the phase transition from solid to liquid
By rearranging this equation, we can express the relationship between melting point and density as:
(dTm/dP) = (T * ΔVfus) / ΔHfus
Where:
– dTm/dP is the change in melting point with respect to pressure
– T is the absolute melting point
– ΔVfus is the change in volume during the phase transition from solid to liquid
– ΔHfus is the latent heat of fusion
This relationship allows us to understand how changes in pressure can affect the melting point of a substance, which is directly related to its density.
Practical Applications of Melting Point and Density
Melting point and density are crucial physical properties that have numerous practical applications in various fields, including:
-
Material Identification: The unique melting point and density of a substance can be used to identify and distinguish different materials, which is particularly useful in forensic science, materials science, and quality control.
-
Phase Transitions and Phase Diagrams: Understanding the melting point and density of a substance is essential for constructing phase diagrams, which are used to predict the behavior of materials under different temperature and pressure conditions.
-
Engineering and Manufacturing: Melting point and density data are crucial in the design and development of various engineering products, such as alloys, ceramics, and polymers, where these properties play a significant role in determining the material’s performance and suitability for specific applications.
-
Thermodynamics and Heat Transfer: Melting point and density are important parameters in the study of thermodynamics and heat transfer, as they influence the behavior of substances during phase changes and the transfer of thermal energy.
-
Geology and Planetary Science: Melting point and density data are essential in the study of geological processes, such as the formation of rocks and minerals, as well as the composition and structure of planets and other celestial bodies.
-
Pharmaceutical and Biomedical Applications: Melting point and density are crucial in the development and characterization of pharmaceutical compounds, as well as in the design of medical devices and implants.
Conclusion
Melting point and density are fundamental physical properties that are essential for understanding the behavior and characteristics of various substances. By mastering these concepts, physics students can gain a deeper understanding of the underlying principles governing the physical world and apply this knowledge to a wide range of scientific and engineering applications.
References
- “Melting Point” on ScienceDirect: https://www.sciencedirect.com/topics/physics-and-astronomy/melting-point
- “Melting Point, Density, and Reactivity of Metals” in the Journal of Chemical Education: https://pubs.acs.org/doi/10.1021/ed078p1054
- “Properties of Matter” on Chemistry LibreTexts: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map:_Chemistry_-_The_Central_Science_%28Brown_et_al.%29/01:_Introduction_-_Matter_and_Measurement/1.03:_Properties_of_Matter
- “Clausius-Clapeyron Equation” on Wikipedia: https://en.wikipedia.org/wiki/Clausius%E2%80%93Clapeyron_relation
- “Density Measurement Techniques” on ScienceDirect: https://www.sciencedirect.com/topics/engineering/density-measurement