In this article, “synthesis reaction example”, different types of synthesis (Williamson synthesis, balanced synthesis and peptide synthesis) example with detailed explanations are discusses briefly.
The examples are-
- Synthesis of ethyl methyl ether
- Synthesis of anisole
- Synthesis of 2-Ethoxynaphthalene
- Synthesis of Phenyl Propyl Ether
- Synthesis of Benzyl-tert butyl ether
- Synthesis of tert-butyl methyl ether
- Synthesis of Ethoxy Benzene
- Synthesis of Cyclopentyl methyl ether
- Synthesis of Water
- Synthesis of Carbon-dioxide
- Synthesis of Ammonia
- Synthesis of Aluminium Oxide
- Synthesis of Iron Sulfide
- Synthesis of Potassium Chloride
- Formation of Rust
- Synthesis of Calcium Carbonate
- Synthesis of Zinc Oxide
- Synthesis of dipeptide (Gly-Ala)
- Solid Phase Peptide Synthesis
What is Synthesis Reaction?
Synthesis reaction is one type of chemical reaction in which two different atoms involve in the reaction, react with each other to form a totally different molecular compound. In most of the synthesis reaction, energy is released from the reaction medium and known as exothermic reaction.

Example of Williamson Synthesis
Synthesis of ethyl methyl ether
Williamson synthesis process is the best method to synthesis ethyl methyl ether (CH3-O- CH2CH3). This reaction proceeds through SN2 pathway. To obtain ethyl methyl ether as the synthesized product, sodium methoxide (CH3ONa) and ethyl chloride (C2H5Cl) reacts with each other. Sodium methoxide acts as nucleophile and attacks the electrophilic centre of ethyl chloride to eliminate the leaving group (Cl–). Ethyl methyl ether is obtained as the Williamson synthesized product.

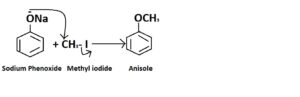
Synthesis of anisole
This ether can also be synthesized by Williamson ether synthesis. To obtain anisole, sodium phenoxide (C5H5ONa) will react with methyl iodide (CH3I) and sodium phenoxide (nucleophile) attacks the electrophilic centre of methyl iodide. Iodide (I–) will be eliminated as it is a good leaving group and anisole is formed.
To know more please check: 12+ Exothermic Reaction Examples: Detailed Explanations
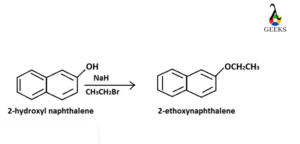
Synthesis of 2-Ethoxynaphthalene
To proceed this reaction, hydroxyl group should be inserted at the 2 position of naphthalene group and reacts with bromoethane. The reaction medium should be basis. Thus, sodium hydride (NaH) is used. Nucleophilic oxygen atom of OH group in naphthalene attacks the CH2 centre of CH3CH2Br and Br– is eliminated as the leaving group.
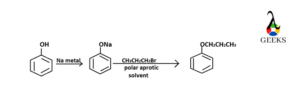
Synthesis of Phenyl Propyl Ether
To synthesis phenyl propyl ether the reactants that are chosen are phenol, sodium metal and n-propyl bromide. Solvent that is used in this synthesis reaction is a polar aprotic solvent. The first step is to react phenol with sodium to form sodium phenoxide (active nucleophile). This nucleophile reacts with n-propyl bromide (electrophile) to synthesize phenyl propyl ether after elimination of bromide ion.
To know more please follow: 11+ First Order Reaction Example: Detailed Explanations
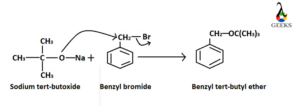
Synthesis of Benzyl-tertbutyl ether
William synthesis pathway is followed for the formation of benzyl-tertbutyl ether. Sodium tert-butoxide and benzyl bromide is taken as the reactants. O– ion from sodium tert-butoxide attacks the electron deficient centre of benzyl bromide Br– is eliminated as the leaving group to form the desired product.
Synthesis of tert-butyl methyl ether
This synthesis process almost similar to the synthesis of benzyl tert-butyl ether. One of the reactants is also same, sodium tert-butoxide and the another reactant is methyl bromide (CH3Br). Tertiary sodium tert butoxide reacts as nucleophile and attacks the methyl carbon center to eliminate bromide ion.
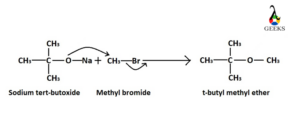
To know more please go through: 10+ Covalent bond types of elements: Detailed Insights And Facts
Synthesis of Ethoxy Benzene
In this process of synthesis of ethoxy benzene, Williamson synthesis process is followed. Sodium ethoxide reacts with phenyl bromide to form ethoxy benzene. O– attacks the electrophilic centre of phenyl bromide and ethoxy benzene is obtained.

Synthesis of Cyclopentyl methyl ether
In this Williamson ether synthesis, cyclopentanol and methyl bromide is reacted with each other in a basic medium. In presence of base, hydrogen in O-H bond is eliminated and O– attacks the methyl bromide to form the cyclopentyl methyl ether.

Example of Balanced Synthesis
Synthesis of Water
Hydrogen and oxygen-these two gases are the two main reactants of this synthesis. Water molecule that is formed is also in gaseous state. Two molecules of hydrogen react with one molecule of oxygen to form water molecule. Dissociation of water is taken place by passing electric through water.
2H2 +O2 = 2H2O
Electrolysis of water results-
- Reduction at cathode: 2H+ (aq) +2e– = H2 (g)
- Oxidation at anode: 2H2O = O2 (g) + 4H+ (aq) + 4e–
- Net balanced equation: 2H2O= 2H2 + O2
Synthesis of Carbon-dioxide
Carbon dioxide is synthesized during the different decay process of various different material and fermentation of sugars. It can be produced by combustion process of wood or other organic materials. Another procedure is to react metal carbonates with dilute acid for the formation of water. For example, carbon dioxide can be synthesized by the reaction between sodium carbonate with dilute HCl.
Synthesis of Ammonia
Haber-Bosch process is the most well known process to synthesize ammonia. High pressure and high temperature is the two most important driving force of ammonia production. It is an exothermic process (del H= -91.8 KJ/mol). Ammonia is widely used as fertilizer.
N2(g) + 3H2 (g) = 2NH3
To know more please check: Disulfide reduction: How, What, Methods and Several Facts
Synthesis of Aluminium oxide
Aluminium hydroxide is the main reactant for the formation of aluminium oxide. Solid Al(OH)3 is decomposed over 11000C and form aluminium oxide (Al2O3). Besides that aluminium is oxidized in presence of oxygen to form aluminium oxide.
2Al(OH)3 = (Al2O3) + 3H2O
4Al (s) + 3O2 (g)= 2Al2O3
Synthesis of Iron Sulfide
Iron after reaction with sulfur forms iron sulfide (pyrrhotite) in presence of heat energy. Iron sulphide (FeS) has totally different physical and chemical properties with respect to two reactants, iron and sulphur. The ratio of iron with sulphur is 1:1. Equal amount of iron is reacted with equal amount of sulphur to form iron sulphide.
Fe + S = FeS
Synthesis of Potassium Chloride
Potassium chloride is basically an ionic salt. It can be synthesized by the reaction bases of potassium like potassium hydroxide (KOH) with strong acid, hydrochloric acid. In this synthesis reaction, strong acid (HCl) is completely neutralized by strong base (KOH). Water is also produced along with the KCl.
KOH (aq) + HCl (aq) = KCl (s) + H2O (liq)
Formation of Rust
Rust is reddish brown iron oxide formed by reacting iron with oxygen. Water or air takes part in this synthesis reaction as catalyst. Chemical formula of rust is Fe2O3.Nh2O and iron oxide hydroxide (FeO (OH),Fe(OH)3).
- Fe(OH)2 = FeO + H2O
- Fe(OH)3 = FeO(OH) + H2O
- 2FeO(OH) = Fe2O3 +H2O
Synthesis of Calcium Carbonate
In this synthesis reaction, calcium oxide (CaO) and carbon dioxide is reacted to form calcium carbonate. At first step, calcium hydroxide is prepared by the reaction between calcium oxide with water. After that Ca(OH)2 is reacted with carbon dioxide and as a product calcium carbonate is obtained.
- CaO +H2O = Ca(OH)2
- Ca(OH)2 +CO2 = CaCO3 +H2O
Synthesis of Zinc Oxide
High temperature is one of the most important driving forces. Zinc vapour is reacted with air (oxygen) at 9100 C. It is mainly an oxidation process and ZnO is produced.
Example of Peptide Synthesis
Synthesis of dipeptide (Gly–Ala)
To synthesis of a dipeptide the following steps should be followed-
- At first alpha amino group of glycine should be blocked by tert-butyloxycarbonylchloride.
- After giving the protection to alpha amino acid of glycine, alanine will react with the previously formed compound.
- Then the tert-butyloxycarbonylchloride group is eliminated by reacting with dilute acid and dipeptide (ala-gly) is obtained as the final product.
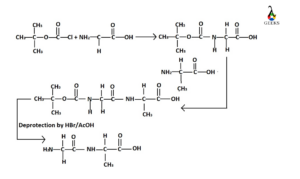
Solid Phase Peptide Synthesis
This synthesis procedure is known as Merrifield synthesis discovered by scientist R.Bruce Merrifield. In this peptide synthesis procedure, homogenous solution is not used for deprotection. This deprotection is carried out at the surface of an insoluble polymer or any solid support.
The carboxyl terminal amino acid is covalently linked with the Merrifield Resin and the length of the peptide chain is increased. Reagents are used to remove the resin with the soluble by products from the peptide chain and at the end desired peptide chain is obtained.

Image Credit: Wikimedia Commons.
Also Read:
- Maillard reaction
- First order reaction example
- Is oxidation a redox reaction
- Light independent reaction in photosynthesis
- Nuclear fission reaction
- Addition reaction example
- Oxidation reaction example
- Decomposition reaction example
- Redox reactions
- Nuclear fusion reaction

Hello,
I am Aditi Ray, a chemistry SME on this platform. I have completed graduation in Chemistry from the University of Calcutta and post graduation from Techno India University with a specialization in Inorganic Chemistry. I am very happy to be a part of the Lambdageeks family and I would like to explain the subject in a simplistic way.
Let’s connect through LinkedIn-https://www.linkedin.com/in/aditi-ray-a7a946202