The surface tension of isopropyl alcohol, also known as 2-propanol or IPA, is a crucial property that governs its behavior in various applications, from its use as a solvent to its role as a cleaning agent and disinfectant. This comprehensive guide delves into the technical details and quantifiable aspects of the surface tension of isopropyl alcohol, providing a valuable resource for science students and professionals alike.
Understanding Surface Tension
Surface tension is a measure of the cohesive forces between the molecules at the surface of a liquid. These forces create a thin, elastic “skin” at the surface, which can support the weight of certain objects and influence the behavior of liquids in various situations. The surface tension of a liquid is typically expressed in units of dynes per centimeter (dyn/cm) or millinewtons per meter (mN/m).
The surface tension of a liquid is determined by the strength of the intermolecular forces between the molecules at the surface. Liquids with strong cohesive forces, such as water, tend to have higher surface tensions, while liquids with weaker intermolecular interactions, like isopropyl alcohol, have lower surface tensions.
The Surface Tension of Isopropyl Alcohol
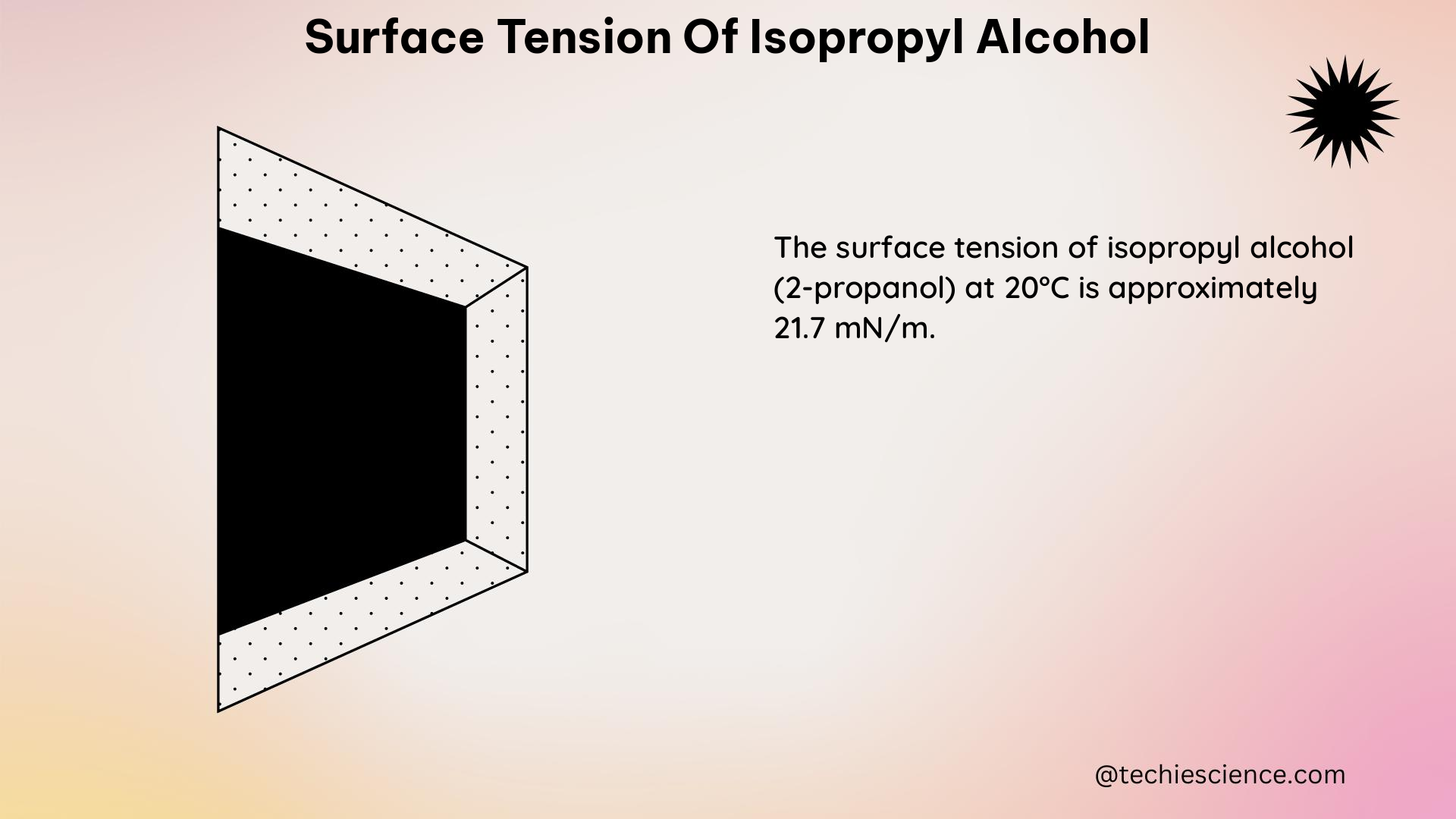
According to the data presented in various studies, the surface tension of isopropyl alcohol at 25°C is 22.62 dynes/cm, with a measurement uncertainty of ±0.3 dynes/cm. This value was determined using the Maximum Bubble Pressure Method A, which is a static method for measuring surface tension.
Factors Affecting Surface Tension
The surface tension of isopropyl alcohol can be influenced by several factors, including:
-
Temperature: The surface tension of isopropyl alcohol decreases as the temperature increases. This is due to the increased thermal energy of the molecules, which weakens the intermolecular forces at the surface.
-
Impurities and Additives: The presence of impurities or other substances in the isopropyl alcohol can alter its surface tension. For example, the addition of surfactants or other solutes can significantly reduce the surface tension of the liquid.
-
Molecular Structure: The specific molecular structure of isopropyl alcohol, with its branched alkyl group and hydroxyl group, contributes to its relatively low surface tension compared to other alcohols, such as ethanol.
Comparison to Other Liquids
Isopropyl alcohol has a significantly lower surface tension compared to water, which has a surface tension of 71.99 dynes/cm at 25°C. This difference is due to the weaker intermolecular forces in isopropyl alcohol, as its molecules have a more compact and less polar structure than water molecules.
The surface tension of isopropyl alcohol is also lower than that of many other common liquids, such as:
- Ethanol: 22.39 dynes/cm at 25°C
- Acetone: 23.32 dynes/cm at 20°C
- Benzene: 28.88 dynes/cm at 20°C
This low surface tension of isopropyl alcohol contributes to its unique properties and applications.
Applications of Isopropyl Alcohol’s Surface Tension
The low surface tension of isopropyl alcohol has several practical implications and applications, including:
-
Solvent Properties: The low surface tension of isopropyl alcohol allows it to effectively wet and penetrate surfaces, making it a useful solvent for various applications, such as cleaning and degreasing.
-
Wetting and Spreading: The low surface tension of isopropyl alcohol enables it to spread and wet surfaces more easily than liquids with higher surface tensions, which is important in applications like printing, coating, and adhesion.
-
Disinfection and Sterilization: Isopropyl alcohol’s low surface tension allows it to effectively penetrate and disinfect surfaces, making it a popular choice for use as a disinfectant and sterilizing agent in medical and industrial settings.
-
Microfluidics and Lab-on-a-Chip: The predictable and well-understood surface tension of isopropyl alcohol makes it a valuable component in microfluidic devices and lab-on-a-chip applications, where the behavior of liquids at the microscale is crucial.
-
Surface Tension Measurements: Isopropyl alcohol is often used as a reference liquid for calibrating and validating surface tension measurement techniques, such as the Maximum Bubble Pressure Method and the Pendant Drop Method.
Theoretical Aspects of Surface Tension
The surface tension of isopropyl alcohol can be understood from a theoretical perspective using various models and equations. One of the most widely used equations for describing the surface tension of liquids is the Young-Laplace equation, which relates the pressure difference across a curved surface to the surface tension and the curvature of the surface.
The Young-Laplace equation can be expressed as:
Δp = γ(1/R1 + 1/R2)
Where:
– Δp is the pressure difference across the curved surface
– γ is the surface tension of the liquid
– R1 and R2 are the principal radii of curvature of the surface
This equation can be used to predict the behavior of isopropyl alcohol in various situations, such as the formation of bubbles, the rise of the liquid in capillary tubes, and the shape of liquid droplets.
Another important theoretical concept related to the surface tension of isopropyl alcohol is the concept of intermolecular forces. The low surface tension of isopropyl alcohol is a result of the relatively weak intermolecular forces between its molecules, which are primarily van der Waals forces and hydrogen bonding. This can be explained using the principles of molecular structure and polarity.
Numerical Examples and Calculations
To further illustrate the surface tension of isopropyl alcohol, let’s consider some numerical examples and calculations:
- Capillary Rise: Suppose we have a glass capillary tube with a radius of 0.5 mm, immersed in isopropyl alcohol at 25°C. Using the Young-Laplace equation and the known surface tension of isopropyl alcohol (22.62 dynes/cm), we can calculate the height of the capillary rise:
h = 2γ cos θ / (ρgr)
Where:
– h is the height of the capillary rise
– γ is the surface tension of isopropyl alcohol (22.62 dynes/cm)
– θ is the contact angle between isopropyl alcohol and the glass (assumed to be 0°)
– ρ is the density of isopropyl alcohol (0.786 g/cm³)
– g is the acceleration due to gravity (980 cm/s²)
– r is the radius of the capillary tube (0.05 cm)
Plugging in the values, we get:
h = 2 × 22.62 × 1 / (0.786 × 980 × 0.05)
h = 5.87 cm
- Bubble Formation: Consider a bubble of isopropyl alcohol forming in the liquid at 25°C. Using the Young-Laplace equation, we can calculate the pressure difference across the bubble’s surface:
Δp = 2γ / R
Where:
– Δp is the pressure difference across the bubble’s surface
– γ is the surface tension of isopropyl alcohol (22.62 dynes/cm)
– R is the radius of the bubble
Assuming the bubble has a radius of 1 mm (0.1 cm), the pressure difference would be:
Δp = 2 × 22.62 / 0.1
Δp = 453.2 dynes/cm²
These examples demonstrate how the surface tension of isopropyl alcohol can be used in various calculations and analyses to predict the behavior of the liquid in different situations.
Conclusion
The surface tension of isopropyl alcohol is a crucial property that governs its behavior and applications in various fields, from solvent use to disinfection and microfluidics. This comprehensive guide has provided a detailed overview of the technical aspects, factors affecting surface tension, theoretical concepts, and numerical examples related to the surface tension of isopropyl alcohol. By understanding the intricacies of this property, science students and professionals can better design, optimize, and utilize isopropyl alcohol in their respective applications.
References
- Surface Tension Lab – Teacher Version | PDF – Scribd
- The Surface Tension of Pure Liquid Compounds
- Surface tension dependences of isopropyl alcohol (IPA), ethanol…
- Lesson 5.2: Surface Tension – American Chemical Society
- Bio 270 Human Physiology Lab 2: Drop – Studocu

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.