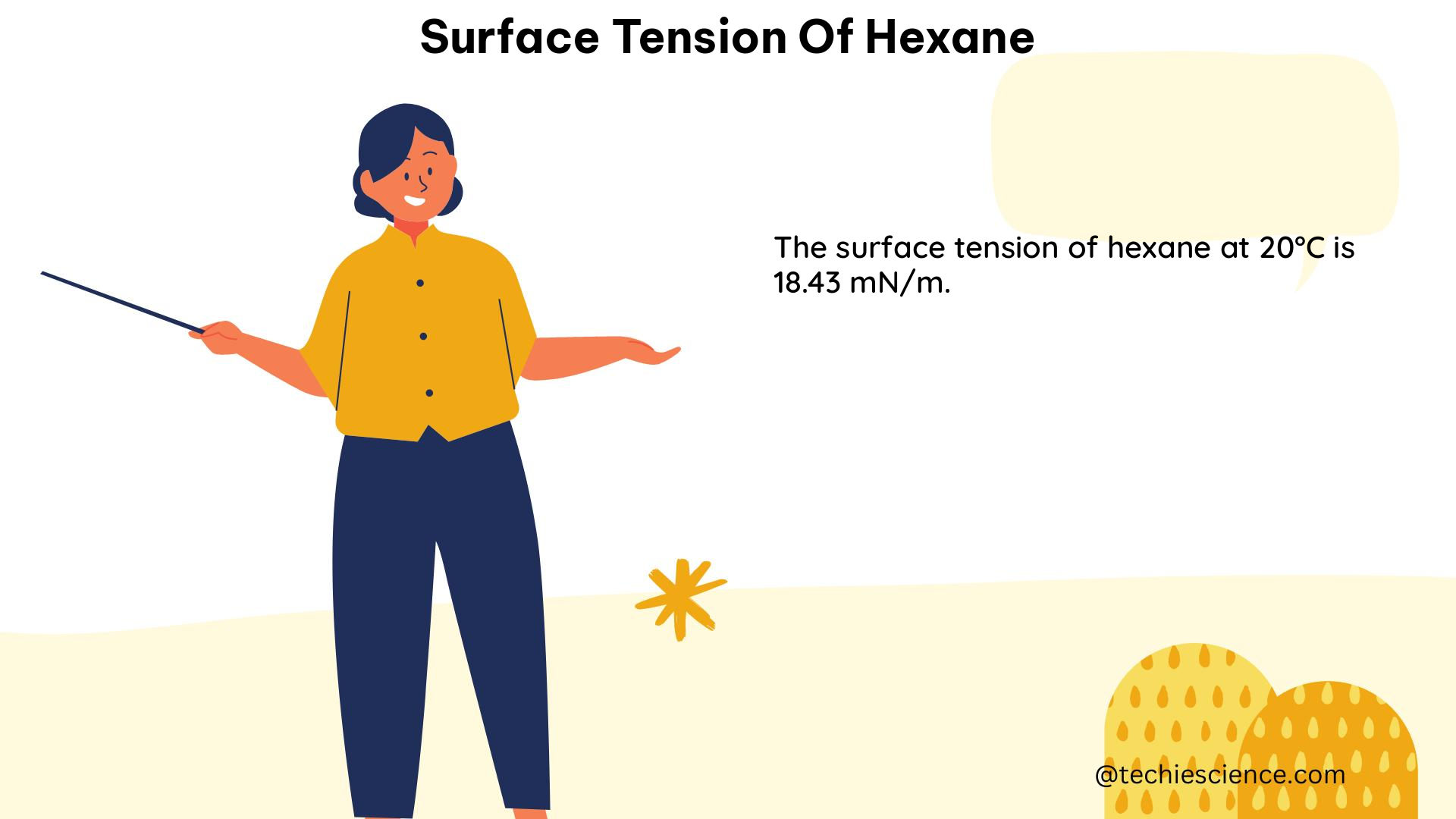
The surface tension of hexane, a widely used organic solvent, is a crucial property that governs various physical and chemical processes. This comprehensive guide delves into the intricacies of measuring, predicting, and understanding the surface tension of hexane, providing a valuable resource for science students and researchers alike.
Understanding the Surface Tension of Hexane
Surface tension is a fundamental property of liquids that arises from the cohesive forces between the molecules at the liquid-air interface. The surface tension of hexane, denoted as γ, is a measure of the work required to increase the surface area of the liquid by a unit amount.
The surface tension of hexane can be expressed in terms of the Young-Laplace equation, which relates the pressure difference across a curved surface to the surface tension and the principal radii of curvature:
ΔP = γ(1/R1 + 1/R2)
where ΔP is the pressure difference, γ is the surface tension, and R1 and R2 are the principal radii of curvature.
Measuring the Surface Tension of Hexane
The surface tension of hexane can be measured using various experimental techniques, each with its own advantages and limitations. Some of the commonly used methods include:
Wilhelmy Plate Method
The Wilhelmy plate method involves suspending a thin, wettable plate (typically made of platinum or glass) at the liquid-air interface and measuring the force required to detach the plate from the surface. The surface tension can be calculated using the following equation:
γ = F / (L cos θ)
where γ is the surface tension, F is the force required to detach the plate, L is the perimeter of the plate, and θ is the contact angle between the plate and the liquid.
Pendant Drop Method
In the pendant drop method, a drop of hexane is formed at the tip of a capillary tube, and the shape of the drop is analyzed to determine the surface tension. The surface tension can be calculated using the following equation:
γ = Δρgd²/H
where γ is the surface tension, Δρ is the density difference between the liquid and the surrounding medium, g is the acceleration due to gravity, d is the diameter of the drop, and H is a dimensionless shape factor.
Capillary Rise Method
The capillary rise method involves measuring the height to which hexane rises in a capillary tube of known diameter. The surface tension can be calculated using the following equation:
γ = (ρgh²) / (2r cos θ)
where γ is the surface tension, ρ is the density of the liquid, g is the acceleration due to gravity, h is the height of the liquid column in the capillary, r is the radius of the capillary, and θ is the contact angle between the liquid and the capillary wall.
Predicting the Surface Tension of Hexane
In addition to experimental measurements, several theoretical models and correlations have been developed to predict the surface tension of hexane. One such model is the van der Waals equation, which can be used to estimate the surface tension of hexane as a function of temperature:
γ = (27/64) * (Tc^2 / Pc) * (1 - T/Tc)^(11/9)
where γ is the surface tension, Tc is the critical temperature, Pc is the critical pressure, and T is the absolute temperature.
Another correlation, developed by Somayajulu, uses the number of carbon atoms in the molecule to predict the surface tension of hexane:
γ = 20.1 - 0.0272 * (Nc - 1)
where γ is the surface tension and Nc is the number of carbon atoms in the molecule.
Factors Affecting the Surface Tension of Hexane
The surface tension of hexane can be influenced by various factors, including:
- Temperature: The surface tension of hexane decreases with increasing temperature, as the cohesive forces between the molecules weaken.
- Pressure: The surface tension of hexane generally increases with increasing pressure, as the molecules are brought closer together.
- Impurities: The presence of impurities, such as other organic compounds or surfactants, can significantly alter the surface tension of hexane.
- Molecular Structure: The surface tension of hexane is influenced by its molecular structure, particularly the number and arrangement of carbon atoms.
Applications of Surface Tension in Hexane-Based Systems
The surface tension of hexane plays a crucial role in various applications, including:
- Wetting and Spreading: The surface tension of hexane determines its ability to wet and spread on different surfaces, which is important in applications such as coatings, printing, and adhesives.
- Emulsion and Foam Formation: The surface tension of hexane influences the stability and behavior of emulsions and foams, which are important in industries like cosmetics, pharmaceuticals, and oil recovery.
- Capillary Action: The surface tension of hexane, along with the contact angle, determines the height to which it can rise in narrow capillaries, which is relevant in microfluidic devices and porous media.
- Interfacial Phenomena: The surface tension of hexane affects the behavior of liquid-liquid and liquid-gas interfaces, which is important in processes like extraction, distillation, and adsorption.
Conclusion
The surface tension of hexane is a fundamental property that governs a wide range of physical and chemical processes. By understanding the measurement techniques, predictive models, and factors affecting the surface tension of hexane, scientists and engineers can optimize the performance of hexane-based systems and develop innovative applications. This comprehensive guide provides a valuable resource for students and researchers working in the fields of chemistry, physics, and engineering.
References
- Adamson, A. W., & Gast, A. P. (1997). Physical Chemistry of Surfaces (6th ed.). Wiley-Interscience.
- Somayajulu, G. R. (1988). A generalized equation for surface tension from the critical temperature. The Journal of Chemical Thermodynamics, 20(6), 659-661.
- Vargaftik, N. B., Volkov, B. N., & Voljak, L. D. (1983). International tables of the surface tension of water. Journal of Physical and Chemical Reference Data, 12(3), 817-820.
- Zeppieri, S., Rodríguez, J., & López de Ramos, A. L. (2001). Interfacial tension of alkane + water systems. Journal of Chemical & Engineering Data, 46(5), 1086-1088.
- Adamson, A. W., & Gast, A. P. (1997). Physical Chemistry of Surfaces (6th ed.). Wiley-Interscience.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.