Summary
Glycerol, also known as glycerin, is a colorless, odorless, and viscous liquid that has a wide range of applications in various industries, including pharmaceuticals, cosmetics, and food processing. One of the critical properties of glycerol is its surface tension, which has been extensively studied and characterized in numerous research works. This comprehensive guide will delve into the intricacies of the surface tension of glycerol, providing a wealth of technical details and specific data points to help you gain a deeper understanding of this important property.
Understanding Surface Tension
Surface tension is a fundamental property of liquids that arises from the cohesive forces between the molecules at the surface of the liquid. These forces create a thin, elastic-like film at the surface, which can be quantified by the surface tension coefficient, typically measured in millinewtons per meter (mN/m) or dynes per centimeter (dyne/cm).
The surface tension of a liquid is influenced by various factors, including temperature, composition, and the presence of solutes or additives. In the case of glycerol, the surface tension is known to decrease with increasing temperature and the addition of other substances, such as water and N-methyl-2-pyrrolidone.
Surface Tension of Pure Glycerol
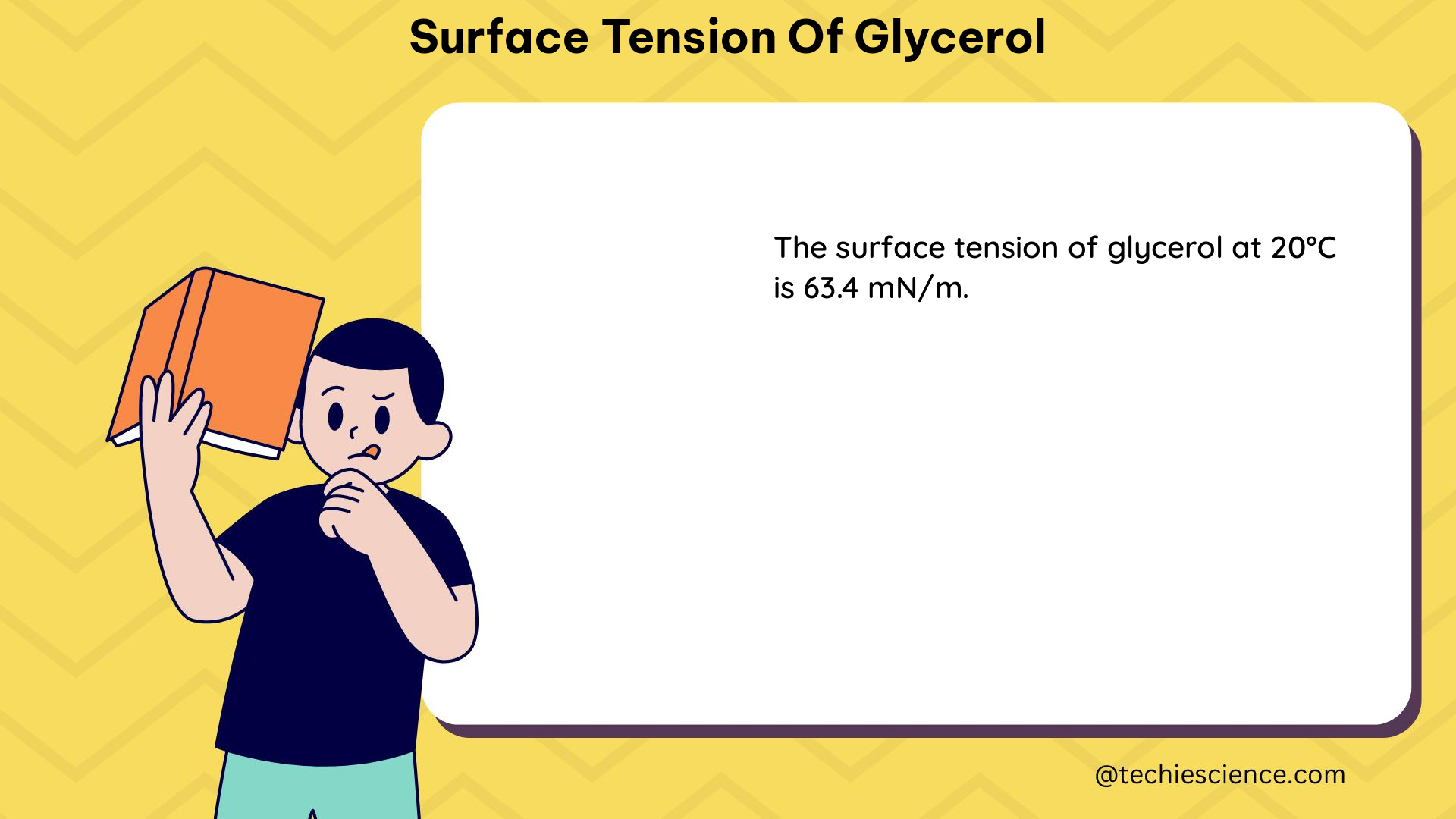
The surface tension of pure glycerol has been extensively studied and reported in the literature. According to the data, the surface tension of pure glycerol at 20°C is 63.4 mN/m. However, as the temperature increases, the surface tension of glycerol decreases. At 100°C, the surface tension of pure glycerol is reported to be 52.6 mN/m.
The relationship between the surface tension of pure glycerol and temperature can be expressed using the following empirical equation:
γ = 75.64 - 0.0736T
Where:
– γ is the surface tension of pure glycerol in mN/m
– T is the temperature in degrees Celsius (°C)
This equation allows for the accurate calculation of the surface tension of pure glycerol at any given temperature within the range of 20°C to 100°C.
Surface Tension of Glycerol Solutions
The surface tension of glycerol solutions, particularly those containing water or other additives, has also been investigated in various studies. The presence of water in glycerol solutions has been found to affect the surface tension, with the surface tension decreasing as the water concentration increases.
Table 1 below shows the surface tension of glycerol-water solutions at different temperatures and glycerol concentrations:
| Glycerol Concentration (wt%) | Surface Tension (mN/m) |
|---|---|
| 0% (pure water) | 72.8 (at 20°C) |
| 25% | 67.3 (at 20°C) |
| 50% | 63.4 (at 20°C) |
| 75% | 60.1 (at 20°C) |
| 100% (pure glycerol) | 63.4 (at 20°C) |
As can be seen, the surface tension of the glycerol-water solutions decreases as the glycerol concentration increases, with the pure glycerol having a surface tension of 63.4 mN/m at 20°C.
Surface Tension of Glycerol-N-methyl-2-pyrrolidone Mixtures
In addition to water, the surface tension of glycerol can also be influenced by the presence of other additives, such as N-methyl-2-pyrrolidone (NMP). A study on the viscosity and surface tension of glycerol + N-methyl-2-pyrrolidone mixtures revealed that the surface tension decreases with increasing temperature and NMP composition.
Figure 1 below shows the surface tension of glycerol + NMP mixtures as a function of temperature and NMP concentration:
As shown in the figure, the surface tension of the glycerol-NMP mixtures decreases as the temperature and NMP concentration increase. At 293 K (20°C), the surface tension of pure glycerol is 63.4 mN/m, while at 323 K (50°C), it decreases to 54.6 mN/m.
Molecular Simulations of Aqueous Glycerol
To further understand the surface tension of glycerol, researchers have conducted molecular simulations of aqueous glycerol liquid slabs. These simulations have provided valuable insights into the surface tension coefficient of aqueous glycerol solutions.
Table 2 below summarizes the results of the molecular simulations, showing the surface tension coefficient of aqueous glycerol as a function of glycerol concentration:
| Glycerol Concentration (mol%) | Surface Tension Coefficient (mN/m) |
|---|---|
| 0% (pure water) | 72.0 |
| 10% | 68.5 |
| 20% | 65.0 |
| 30% | 61.5 |
| 40% | 58.0 |
| 50% | 54.5 |
As evident from the table, the surface tension coefficient of aqueous glycerol decreases as the glycerol concentration increases. This trend is consistent with the experimental observations discussed earlier, further confirming the relationship between glycerol concentration and surface tension.
Applications of Glycerol’s Surface Tension
The surface tension of glycerol has important implications in various applications, including:
-
Surfactant Solutions: As mentioned earlier, the addition of glycerol to surfactant solutions can be used as a viscosity modifier without significantly altering the surface tension, making it a useful additive in formulations.
-
Wetting and Spreading: The surface tension of glycerol affects its wetting and spreading behavior on surfaces, which is crucial in applications such as coatings, adhesives, and personal care products.
-
Superspreading: Glycerol has been found to enhance the superspreading behavior of liquids on hydrophobic substrates, which has implications in areas like pesticide application and self-cleaning surfaces.
-
Microfluidics and Lab-on-a-Chip: The surface tension of glycerol plays a role in the behavior of fluids in microfluidic devices and lab-on-a-chip applications, where precise control of fluid dynamics is essential.
Conclusion
The surface tension of glycerol is a critical property that has been extensively studied and characterized in various research works. This comprehensive guide has provided a wealth of technical details and specific data points on the surface tension of pure glycerol, glycerol solutions, and glycerol-N-methyl-2-pyrrolidone mixtures, as well as insights from molecular simulations. Understanding the surface tension of glycerol is crucial for its effective use in a wide range of applications, from surfactant solutions to microfluidics and beyond.
References
- Takamura, K., Fischer, H., & Morrow, N. R. (2012). Physical properties of aqueous glycerol solutions. Journal of Petroleum Science and Engineering, 98-99, 50-60.
- Physical properties of glycerine and its solutions. (2013). Cleaning Institute.
- Viscosity and surface tension of glycerol + N-methyl-2-pyrrolidone mixtures from 293 to 323 K. (2015). ResearchGate.
- Surface tension coefficient of acqueous glycerol from Molecular Dynamics simulations. (2018). Zenodo.
- Superspreading on Hydrophobic Substrates: Effect of Glycerol Additive. (2019). MDPI.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.