The solubility of lead nitrate in water is a crucial parameter in various chemical and industrial applications. At 30°C, the solubility of lead nitrate is 66 grams per 100 grams of water, while at 50°C, it increases to 85 grams per 100 grams of water. This means that at 30°C, 66% of lead nitrate can be dissolved in water, and at 50°C, 85% of lead nitrate can be dissolved in water. The solubility of lead nitrate at 10°C is 50.0 grams per 100 grams of water.
Understanding the Factors Affecting Solubility
The solubility of a solute in a solvent is influenced by several factors, including temperature, the presence of other solutes, pressure, and pH.
Temperature and Solubility
As mentioned earlier, the solubility of lead nitrate in water increases with an increase in temperature. This is due to the kinetic energy of the solvent molecules, which increases as the temperature rises. The higher kinetic energy allows the solvent molecules to break the intermolecular forces between the solute molecules more easily, resulting in an increase in solubility.
The relationship between temperature and solubility can be expressed using the following equation:
S = S0 + k(T - T0)
Where:
– S is the solubility of the solute at temperature T
– S0 is the solubility of the solute at the reference temperature T0
– k is the temperature coefficient of solubility
By using this equation, you can calculate the solubility of lead nitrate at any given temperature within the range of the available data.
Common Ion Effect and Solubility
The solubility of a solute in a solvent can also be affected by the presence of other solutes in the solution, a phenomenon known as the common ion effect. If a salt containing a lead ion is added to a solution of lead nitrate, the solubility of lead nitrate will decrease due to the common ion effect.
The common ion effect can be explained using the equilibrium constant (Ksp) for the solubility of lead nitrate. The Ksp for lead nitrate is given by the following equation:
Ksp = [Pb2+][NO3-]2
When a salt containing a lead ion is added to the solution, the concentration of Pb2+ ions increases, which shifts the equilibrium to the left, decreasing the solubility of lead nitrate.
Pressure and Solubility
The solubility of a solute in a solvent can also be affected by pressure, although this factor is usually less significant compared to temperature and the presence of other solutes. In general, an increase in pressure will slightly increase the solubility of a solute, as the higher pressure can compress the solvent molecules, allowing more solute to dissolve.
pH and Solubility
The pH of the solution can also influence the solubility of a solute. In the case of lead nitrate, the solubility may be affected by the pH of the solution, as lead ions can form different complexes depending on the pH. However, the effect of pH on the solubility of lead nitrate is typically less significant compared to the other factors mentioned.
Practical Applications of Lead Nitrate Solubility
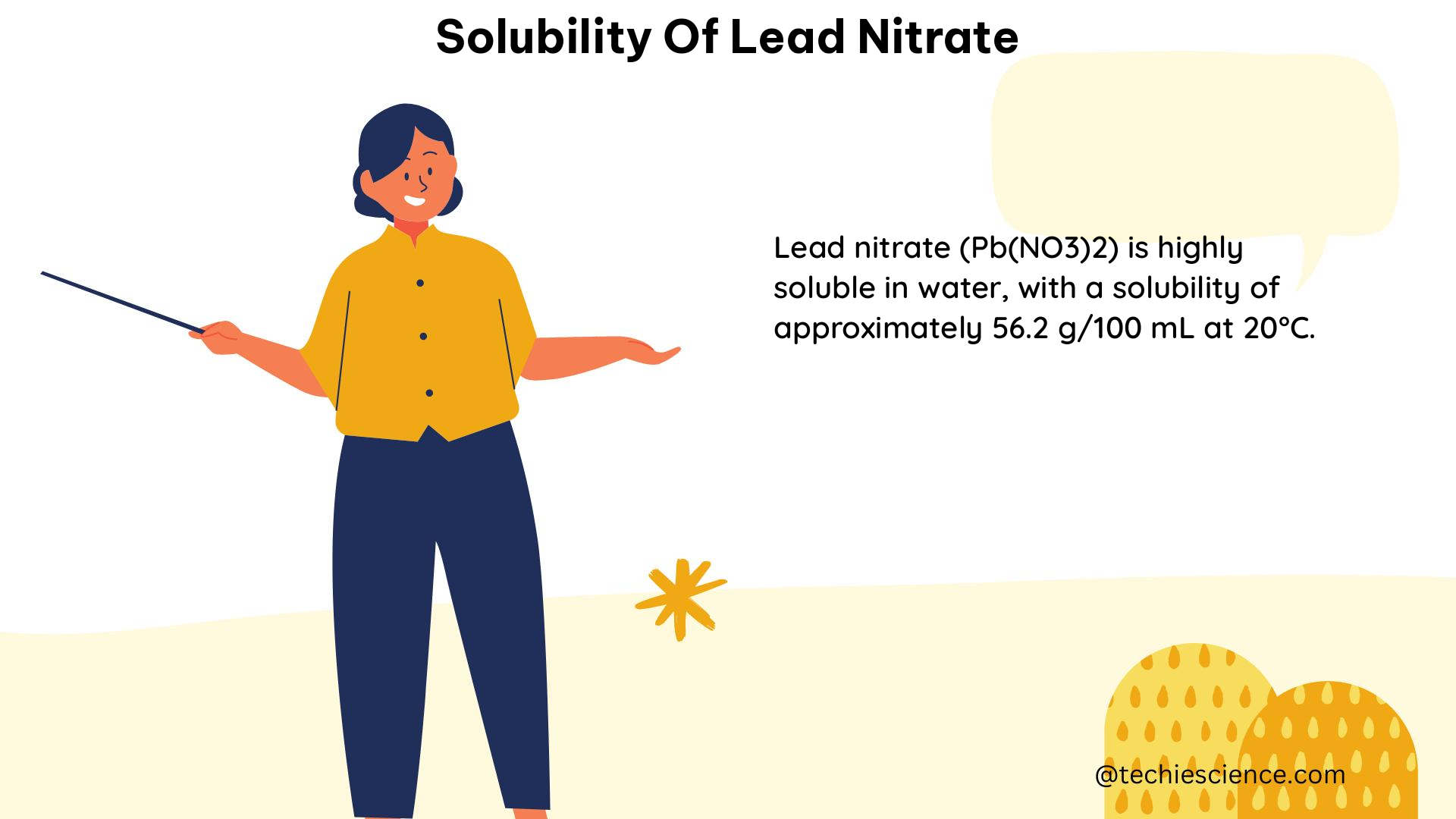
The solubility of lead nitrate in water has various practical applications in different fields, including:
-
Chemical Industry: Lead nitrate is used in the production of pigments, dyes, and other chemical compounds. The solubility of lead nitrate is crucial in controlling the concentration and purity of these products.
-
Environmental Monitoring: Lead nitrate is a common contaminant in water sources, and its solubility is important in understanding the transport and fate of lead in the environment. Knowing the solubility of lead nitrate can help in the development of effective water treatment and remediation strategies.
-
Analytical Chemistry: Lead nitrate is used as a standard in various analytical techniques, such as atomic absorption spectroscopy and inductively coupled plasma mass spectrometry, to quantify the concentration of lead in samples. The solubility of lead nitrate is essential in preparing accurate standard solutions for these analyses.
-
Electroplating: Lead nitrate is used in the electroplating process to deposit lead coatings on various metal surfaces. The solubility of lead nitrate in the plating solution affects the efficiency and quality of the electroplating process.
-
Pyrotechnics: Lead nitrate is used in the production of some pyrotechnic compositions, such as smoke grenades and flares. The solubility of lead nitrate is important in controlling the composition and performance of these pyrotechnic devices.
Experimental Determination of Lead Nitrate Solubility
The solubility of lead nitrate in water can be determined experimentally using various techniques, such as:
-
Gravimetric Method: In this method, a known mass of lead nitrate is added to a fixed volume of water, and the solution is allowed to reach equilibrium. The solution is then filtered, and the mass of the undissolved lead nitrate is determined. The solubility can be calculated by dividing the mass of the dissolved lead nitrate by the mass of the water.
-
Spectrophotometric Method: This method involves the use of a spectrophotometer to measure the absorbance of the lead nitrate solution at a specific wavelength. The absorbance is then related to the concentration of lead nitrate in the solution using a calibration curve.
-
Potentiometric Titration: In this method, the lead nitrate solution is titrated with a standard solution of a complexing agent, such as EDTA. The endpoint of the titration is detected using a potentiometric electrode, and the solubility of lead nitrate can be calculated from the volume of the titrant used.
-
Conductometric Method: This method relies on the measurement of the electrical conductivity of the lead nitrate solution. The conductivity is related to the concentration of the dissolved ions, which can be used to calculate the solubility of lead nitrate.
Regardless of the experimental method used, it is essential to carefully control the experimental conditions, such as temperature, pH, and the presence of other ions, to ensure accurate and reproducible results.
Numerical Examples and Data Points
Here are some numerical examples and data points related to the solubility of lead nitrate in water:
- Solubility at Different Temperatures:
- At 10°C, the solubility of lead nitrate is 50.0 grams per 100 grams of water.
- At 30°C, the solubility of lead nitrate is 66 grams per 100 grams of water.
-
At 50°C, the solubility of lead nitrate is 85 grams per 100 grams of water.
-
Solubility Product Constant (Ksp) of Lead Nitrate:
-
The Ksp of lead nitrate at 25°C is 4.4 × 10^-13.
-
Molar Solubility of Lead Nitrate:
-
At 25°C, the molar solubility of lead nitrate is 0.0066 mol/L.
-
Solubility Equilibrium Equation:
-
The solubility equilibrium for lead nitrate in water can be represented as:
Pb(NO3)2(s) ⇌ Pb2+(aq) + 2 NO3-(aq) -
Enthalpy of Dissolution:
-
The enthalpy of dissolution for lead nitrate in water is 17.1 kJ/mol.
-
Density of Saturated Lead Nitrate Solution:
-
The density of a saturated lead nitrate solution at 20°C is 1.853 g/mL.
-
Molar Mass of Lead Nitrate:
- The molar mass of lead nitrate (Pb(NO3)2) is 331.21 g/mol.
These data points and numerical examples can be used to further understand and analyze the solubility of lead nitrate in water, as well as to perform various calculations and problem-solving exercises related to this topic.
Conclusion
The solubility of lead nitrate in water is a crucial parameter that is influenced by various factors, including temperature, the presence of other solutes, pressure, and pH. Understanding the solubility of lead nitrate is essential in various applications, such as the chemical industry, environmental monitoring, analytical chemistry, electroplating, and pyrotechnics.
By exploring the factors affecting solubility, the practical applications of lead nitrate solubility, and the experimental methods for determining solubility, this comprehensive guide provides a valuable resource for students, researchers, and professionals working in fields related to the chemistry and applications of lead nitrate.
References:
- Explanation of Lead Nitrate Solubility at 30°C and 50°C
- Solubility of Lead Nitrate at 100°C
- Testing for Lead and Nitrates in Water
- Solubility Product Constant (Ksp) of Lead Nitrate
- Enthalpy of Dissolution for Lead Nitrate
- Density of Saturated Lead Nitrate Solution

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.