Summary
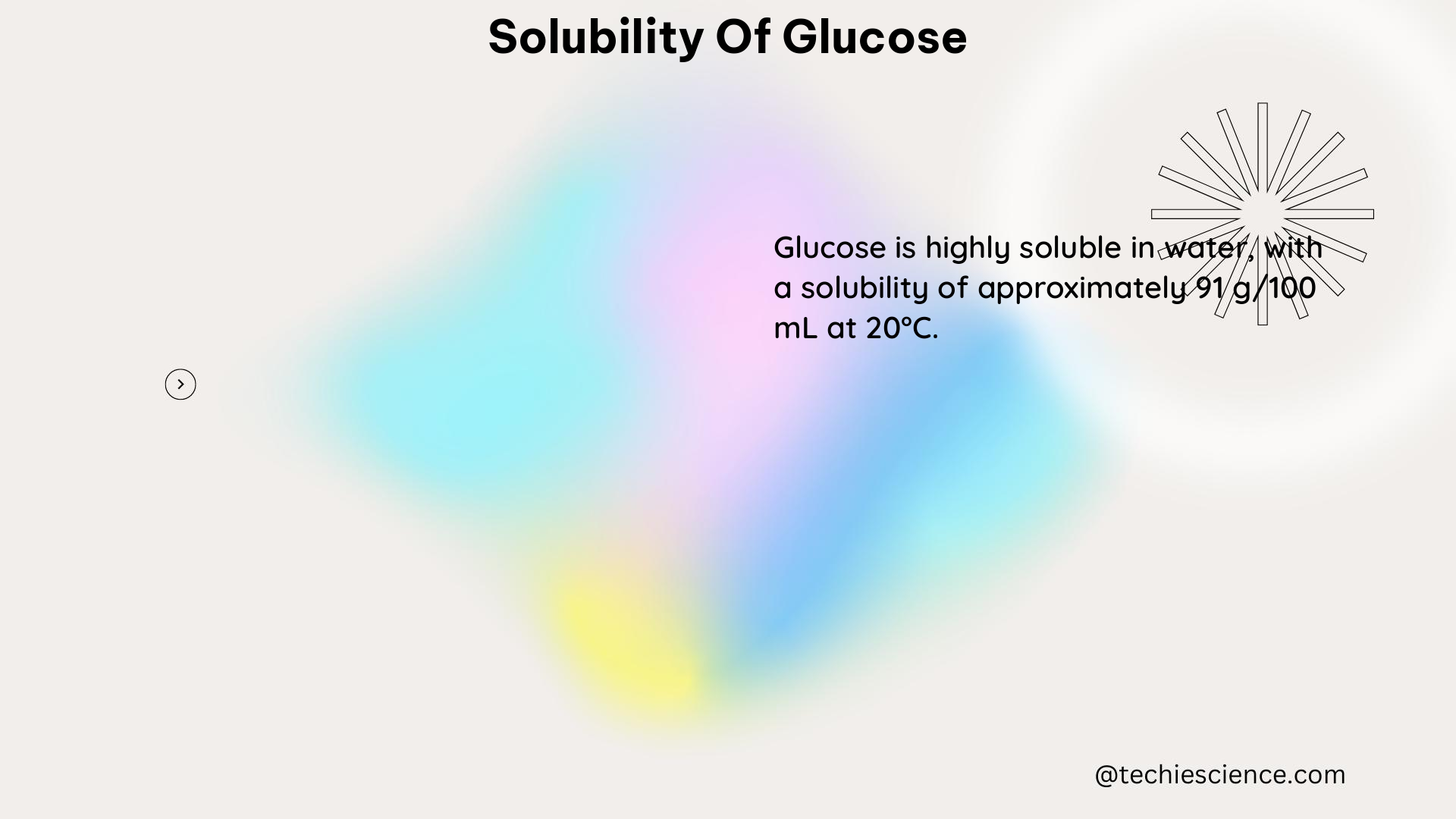
Glucose, a monosaccharide and the primary source of energy for many organisms, has a solubility of approximately 1.0 g/mL in water at room temperature. This means that 1 gram of glucose can dissolve in 1 milliliter of water. The solubility of glucose in ethanol/water mixtures has also been extensively studied, and it is found to be high, making it useful for investigating the glucose crystallization process in these mixtures.
Understanding Glucose Solubility
Factors Affecting Glucose Solubility
The solubility of glucose in water is influenced by several factors, including:
-
Temperature: The solubility of glucose increases with increasing temperature. At higher temperatures, the kinetic energy of the glucose molecules increases, allowing them to overcome the intermolecular forces and dissolve more readily in water.
-
Pressure: The solubility of glucose is generally not significantly affected by changes in pressure, as it is a non-volatile solute.
-
Molecular Structure: The presence of hydroxyl (-OH) groups in the glucose molecule allows for the formation of hydrogen bonds with water molecules, enhancing the solubility of glucose in aqueous solutions.
-
Polarity: Glucose is a polar molecule, which means it has a uneven distribution of electrons, making it soluble in polar solvents like water.
Glucose Solubility in Water
The solubility of glucose in water at 20°C (68°F) is approximately 1.0 g/mL. This means that 1 gram of glucose can dissolve in 1 milliliter of water at room temperature. The solubility of glucose in water can be expressed using the following equation:
Solubility (g/mL) = Mass of Glucose (g) / Volume of Water (mL)
For example, if you have 5 grams of glucose and you want to dissolve it in water, you would need 5 mL of water to fully dissolve the glucose.
Glucose Solubility in Ethanol/Water Mixtures
The solubility of glucose in ethanol/water mixtures has been extensively studied, and it is found to be high. This makes it useful for investigating the glucose crystallization process in these mixtures.
The solubility of glucose in ethanol/water mixtures can be expressed using the following equation:
Solubility (g/mL) = Mass of Glucose (g) / Volume of Ethanol/Water Mixture (mL)
The specific solubility values for glucose in different ethanol/water ratios can be found in the references provided at the end of this article.
Measuring Glucose Solubility
Standardized Protocols for Measuring Glucose Solubility
The protocol for total soluble sugar quantification from ethanolic plant extracts via the sulfuric phenol method adapted for 96-well plates provides a detailed and standardized method for measuring the soluble sugars, including glucose, in plant tissue extracts.
Materials and Equipment
- Microcentrifuge tubes
- 96-well plate
- Pipettes and tips
- Sulfuric acid
- 5% w/v phenol solution
- UV-Vis plate reader
Procedure
- Prepare glucose standards in microcentrifuge tubes by pipetting the appropriate amounts of 1 mg/mL Glucose standard and distilled water.
- Extract and purify total soluble sugars from plant tissue per the Extraction of Non-Structural Carbohydrates (Total Soluble Sugars + Starch) in Plant Tissues protocol.
- Prepare the sulfuric phenol reagent by mixing sulfuric acid and 5% w/v phenol.
- Add the sulfuric phenol reagent to each well of the 96-well plate, followed by the addition of the glucose standards or plant tissue extracts.
- Incubate the plate in a water bath for 20 minutes.
- Measure the absorbance of each well at 490 nm using a UV-Vis plate reader.
- Create a standard curve using the glucose standards and calculate the concentration of total soluble sugars, including glucose, in the plant tissue extracts.
This protocol provides a standardized and accurate method for measuring the solubility of glucose in plant tissue extracts.
Factors Affecting Glucose Solubility Measurement
When measuring the solubility of glucose, it is important to consider the following factors that can affect the accuracy and precision of the measurements:
-
Sample Preparation: Proper extraction and purification of the plant tissue samples are crucial to ensure accurate glucose measurements.
-
Reagent Quality: The quality and concentration of the sulfuric phenol reagent can impact the color development and absorbance measurements.
-
Incubation Time and Temperature: Adherence to the specified incubation time and temperature is essential for consistent color development and accurate glucose quantification.
-
Spectrophotometric Measurements: The accuracy of the UV-Vis plate reader and the proper calibration of the instrument can affect the absorbance measurements.
-
Standard Curve: The preparation and use of an accurate glucose standard curve is crucial for converting absorbance values to glucose concentrations.
By carefully controlling these factors, researchers can obtain precise and accurate measurements of glucose solubility in plant tissue extracts.
Practical Applications of Glucose Solubility
The understanding of glucose solubility has various practical applications, including:
-
Food and Beverage Industry: The solubility of glucose is important in the formulation and processing of food and beverage products, such as jams, syrups, and sports drinks.
-
Pharmaceutical Industry: Glucose is used as a sweetener and excipient in various pharmaceutical formulations, and its solubility is a critical parameter in drug development and manufacturing.
-
Biotechnology and Fermentation: The solubility of glucose is crucial in fermentation processes, where it serves as a carbon source for microbial growth and product formation.
-
Plant Physiology and Ecology: Understanding the solubility of glucose in plant tissues is essential for studying the storage and mobilization of non-structural carbohydrates, which are important for plant growth, development, and stress responses.
-
Analytical Chemistry: The standardized protocols for measuring glucose solubility, such as the sulfuric phenol method, are widely used in analytical chemistry laboratories for the quantification of soluble sugars in various samples.
By understanding the factors that influence glucose solubility and the standardized methods for its measurement, researchers and professionals can optimize processes, develop new products, and conduct more accurate analyses in a wide range of applications.
References
- Standardized protocols and procedures can precisely and accurately measure non-structural carbohydrates (NSCs) in plant samples. NCBI, 2018. Link
- Total Soluble Sugar Quantification from Ethanolic Plant Extracts. Protocols.io, 2021. Link
- Solubility of d-Glucose in Water and Ethanol/Water Mixtures. ResearchGate, 2012. Link
- Solubility of anhydrous-glucose in ethanol/water mixture. ResearchGate, 2011. Link
- Lab: Solubility Assignment: Reflect on the Lab. Quizlet, 2021. Link

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.