Radioactive decay occurs when an unstable nucleus releases energy through radiation and becomes a stable nuclei. Radioactive disintegration can be in the form of alpha particles, beta particles, gamma rays, positron emission, electron capture,etc. Few radioactive decay examples are discussed in detail in this article.
- Alpha decay of Uranium-238 nucleus
- Beta decay of Thorium-234 nucleus
- Alpha decay of Polonium-210 nucleus
- Beta decay of Iodine-131 nucleus
- Gamma decay of Cobalt-60 nucleus
- Positron emission of Oxygen-15 nucleus
- Electron capture of Potassium-40
- Alpha decay of Uranium-234 nucleus
- Alpha decay of Thorium-230 nucleus
- Alpha decay of Radium-226
- Alpha decay of Polonium-218 nucleus
- Alpha decay of Radon-222 nucleus
- Beta decay of Lead-214
- Beta decay of Bismuth-214
- Alpha decay of Polonium-214
- Beta decay of Lead-210
- Beta decay of Bismuth-210
Alpha decay of Uranium-238 nucleus
Uranium-238, most common isotope of Uranium, undergoes alpha decay and forms Thorium-234. During this reaction, unstable uranium-238 nucleus loses 2 protons and 2 neutrons to form thorium-234. The alpha particle can be regarded as a Helium nucleus.
The alpha particles are less penetrating than other forms of radiation. Sometimes weak gamma rays are also emitted during the decay process. Of all the radioactive disintegration processes, alpha decay is the least dangerous.
The radioactive decay can be shown as
Image Credits: Wikimedia Commons
Beta decay of Thorium-234 nucleus
The thorium-234 nuclide undergoes beta decay by releasing an electron and protactinium-234 is formed. This kind of beta decay is known as beta minus decay since an energetic negative electron is released.
The decay process can be depicted by the following balanced equation:
The
represents anti-neutrino.
As mentioned earlier, the decay of thorium-234 to protactinium-234 is a beta minus decay. The underlying process is that a neutron breaks into a proton plus an electron; and the electron is released out of the nucleus while the proton stays inside the nucleus.
Alpha decay of Polonium-210 nucleus
Polonium is one of the naturally occurring radioactive element and occurs in relatively very low concentrations in the Earth’s crust.
Polonium-210, stable isotope of polonium, decays into a stable nucleus lead-206 by emitting an alpha particle. The alpha particles emitted from polonium-210 are capable of ionizing adjacent air which in turn, neutralizes static electricity on the surfaces that are in contact with air.
The decay process can be represented as follows:
Polonium-210 finds applications in many static eliminators which are essentially used to eliminate static electricity in certain devices due to the property of the emitted alpha particles.
Beta decay of Iodine-131 nucleus
Iodine-131 nucleus undergoes beta decay and forms a stable xenon-131 nucleus. This is also a beta-minus decay.
The decay reaction is as given below:
Since both beta particle and gamma ray are emitted, it is also known as a beta-gamma emitter. This makes it useful in the field of nuclear medicine.
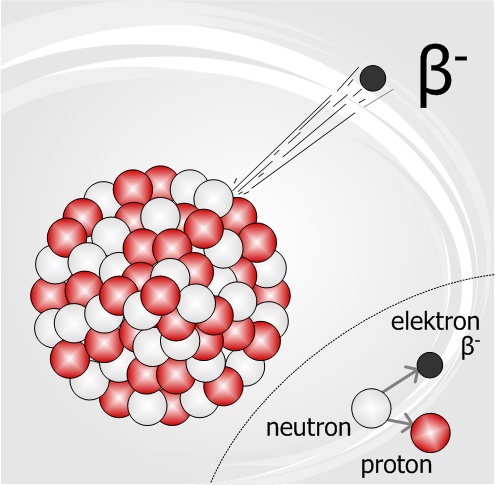
Image Credits: Wikimedia Commons
Gamma decay of Cobalt-60 nucleus
Cobalt-60 is a radioactive isotope of cobalt but not naturally occurring.
The actual reaction takes place by the beta decay of Cobalt-60 to produce stable Nickel-60 and this nucleus emits two gamma rays.
The reaction can be represented as:
Being a high intensity gamma emitter, Cobalt-60 has several applications like radiation source for radiotherapy, food irradiation, pest insect sterilization, and so on.
Positron emission of Oxygen-15 nucleus
The neutron to proton ratio is a key factor that determines the stability of any nucleus. Radioactive decays takes place to stabilize the nucleus.
In oxygen-15, the number of neutrons is 7 which is less than the number of protons i.e., 8. Hence it undergoes positron emission and nitrogen-15 is formed. Positron emission is otherwise known as beta plus decay.
This is what happens in a positron emission:
The reaction of beta plus decay of oxygen-15 can be represented as:
Electron capture of Potassium-40
Potassium-40 is an example for a naturally occurring radioactive isotope of potassium, but relatively in very small fraction, around 0.012%.
Electron capture is a radioactive decay process when there is an abundance of protons in the nucleus compared to neutrons in addition to the insufficient energy for positron emission.
During an electron capture, nucleus captures an atomic electron and hence proton is transformed to neutron.
The electron capture of potassium-40 is
Image Credits: Wikimedia Commons
Alpha decay of Uranium-234 nucleus
The uranium-234 is an indirect decay product of uranium-238 and is immediately converted to thorium-230 by alpha decay.
Emitted alpha particle is comparatively less penetrative and thorium-230 is formed.
The decay reaction is:
Alpha decay of Thorium-230 nucleus
Thorium-230 is one of the naturally occurring radioactive isotopes of thorium.
Thorium-230 is a part of the uranium decay series and radium-226 is the product of radioactive decay of this thorium nucleus. Alpha particles are emitted during the process.
The alpha decay can be shown as:
Thorium-230, being a decay product of Uranium-238, is found in uranium deposits and in uranium mill tailings.
Alpha decay of Radium-226
Radium is an alpha particle radiator, a decay product of uranium-238 decay series and is present in rocks and soils in different amounts.
Radium-226 yields radon-222, a radioactive inert gas upon alpha particle emission.
The decay reaction is:
Radium is highly radioactive as it is about one million times more radioactive than uranium and the decay product, radon is used nowadays to treat various forms of cancer.
Image Credits: Wikimedia Commons
Alpha decay of Polonium-218 nucleus
Polonium-218 disintegrates mainly by alpha decay although it is observed that beta emission takes place in fewer amounts in some cases.
Alpha disintegration of polonium-218 can be represented by the following reaction:
Alpha decay of Radon-222 nucleus
Radon-222, a highly radioactive gaseous element, is radon’s most stable isotope. Radon-222 is one of the leading causes of lung cancer as it is a gas and radioactive.
Radon-222 undergoes alpha disintegration and polonium-218 is produced.
The disintegration reaction is:
Radon is a major cancer-causing agent as it can be inhaled and before its exhalation, it undergoes decay producing alpha particles and/or gamma rays which can damage our cells. Hence radon can cause lung cancer.
Beta decay of Lead-214
Lead-214 undergoes beta emission and forms Bismuth-214. The type of beta decay is beta minus decay.
The radioactive process can be shown as:
Beta decay of Bismuth-214
Bismuth-214 undergoes beta disintegration to form Polonium-214 nuclide. The decay process is beta minus decay.
The underlying reaction is:
Alpha decay of Polonium-214
The alpha decay of polonium-214 yields lead-210.
The representation of the decay reaction is:
Beta decay of Lead-210
Lead-210 is a naturally occurring radioactive nuclide of the uranium decay series.
A beta minus decay of lead-210 yields bismuth-210. This process is accompanied by emission of energy through gamma rays.
The reaction for the beta minus disintegration can be represented in the following way:
Beta decay of Bismuth-210
Bismuth-210 undergoes beta disintegration and forms polonium-210.
The beta minus decay can be depicted as follows:
In nature, polonium is found more concentrated in tobacco. Being an alpha emitter when tobacco is smoked, polonium gets inhaled leading to the damage of cells due to the emitted alpha particles from polonium.
Conclusion
In this article, several radioactive decay examples have been discussed in detail. Eventhough exposure to radiation is harmful in several contexts; some radioactive decay processes find application in medical field, especially to treat cancer. Apart from medical applications, several industrial processes make use of decay process depending on the needs.
Also Read:
- Why is vitamin d often called the sunshine vitamin
- Where do phenomena like halos and sun dogs come from in meteorology
- Amplitude
- Movable pulley examples
- Galaxy definition formation 5 interesting facts
- Is malleability a physical property
- Fixed pulley examples
- Loudness and amplitude
- Is brass malleable
- Is hardness a physical property
Hello, I am Deeksha Dinesh, currently pursuing post-graduation in Physics with a specialization in the field of Astrophysics. I like to deliver concepts in a simpler way for the readers.