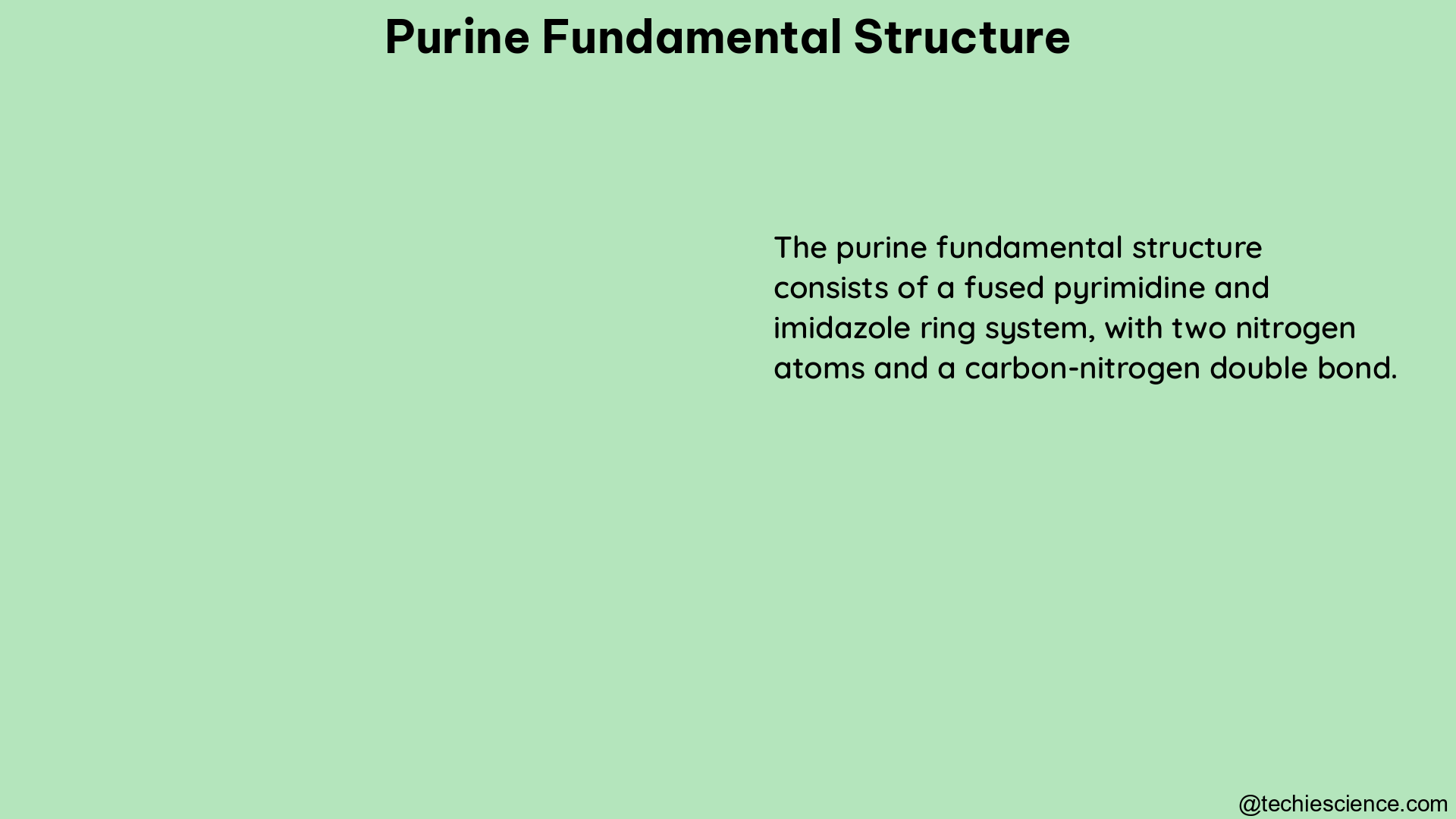
The purine ring is a fundamental structure in biochemistry, composed of a pyrimidine ring fused to an imidazole ring. Purines, including adenine and guanine, are essential components of nucleic acids, such as DNA and RNA, where they pair with pyrimidines via hydrogen bonds. The structure of purines is crucial for the stability and function of these molecules.
The Molecular Structure of Purines
Purines are heterocyclic aromatic organic compounds with a characteristic fused-ring structure. The purine ring consists of two rings: a pyrimidine ring (6-membered) and an imidazole ring (5-membered). This unique structure is responsible for the diverse biological functions of purines.
Atomic Composition and Molecular Weight
The purine ring is composed of 10 atoms: 5 carbon atoms, 4 nitrogen atoms, and 1 hydrogen atom. The molecular weight of the purine ring is approximately 120 Daltons (Da), which is the sum of the atomic weights of its constituent atoms.
Table 1: Atomic Composition and Molecular Weight of Purines
| Purine | Molecular Formula | Molecular Weight (Da) |
|---|---|---|
| Adenine | C₅H₅N₅ | 135.13 |
| Guanine | C₅H₅N₅O | 151.13 |
| Hypoxanthine | C₅H₄N₄O | 136.11 |
| Xanthine | C₅H₄N₄O₂ | 152.11 |
As shown in the table, the molecular weights of individual purines vary due to the presence of additional functional groups, such as the amino group in adenine and the keto group in guanine.
Hydrogen Bonding and Nucleic Acid Formation
Purines play a crucial role in the formation of nucleic acids, such as DNA and RNA. Adenine and guanine form specific hydrogen-bonding patterns with the pyrimidines, cytosine and thymine (in DNA) or uracil (in RNA), respectively. This base pairing is essential for the stability and structure of nucleic acids.
- Adenine pairs with thymine (in DNA) or uracil (in RNA) through two hydrogen bonds.
- Guanine pairs with cytosine through three hydrogen bonds.
The specific hydrogen-bonding patterns between purines and pyrimidines are essential for the double-helix structure of DNA and the secondary structure of RNA, which are fundamental to their biological functions.
Analytical Techniques for Purine Quantification
Purines can be quantified using various analytical techniques, including high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) spectroscopy.
High-Performance Liquid Chromatography (HPLC)
HPLC is a widely used method for the separation, identification, and quantification of purines in biological samples, such as food, plasma, and tissues. Purines are separated based on their chemical and physical properties, such as charge, hydrophobicity, and size, and then detected using various detection methods, such as ultraviolet (UV) absorbance, fluorescence, and electrochemical detection.
HPLC Method Validation and Applications
- A study published in the Journal of Separation Science reported a method for the simultaneous determination of four purines (adenine, guanine, hypoxanthine, and xanthine) in seafood using HPLC with UV detection. The method was validated using spiked samples and showed good linearity, accuracy, and precision, with limits of detection (LOD) and quantification (LOQ) in the range of 0.02-0.15 μg/g and 0.06-1.02 μg/g, respectively.
- Another study published in the Journal of Chromatography A reported a method for the determination of purine contents in different parts of pork and beef using HPLC with UV detection. The method was validated using standard solutions and showed good linearity, accuracy, and precision, with LOD and LOQ values of 0.005-0.01 μg/mL and 0.015-0.03 μg/mL, respectively.
Mass Spectrometry (MS)
MS is another powerful tool for the identification and quantification of purines in biological samples. It offers high sensitivity and specificity, allowing for the detection and quantification of purines at very low concentrations.
HPLC-MS/MS Method Validation and Applications
- A study published in the Journal of Chromatography B reported a method for the determination of purine and pyrimidine bases, nucleosides, and their degradation products in bovine blood plasma using HPLC-MS/MS. The method was validated using spiked samples and showed good linearity, accuracy, and precision, with LOD and LOQ values in the range of 0.01-0.1 ng/mL and 0.03-0.3 ng/mL, respectively.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR spectroscopy is another analytical technique that can be used to identify and quantify purines in biological samples. NMR provides information about the chemical environment and structure of purines, allowing for their identification and quantification.
Importance of Purine Quantification
The quantifiable data on purine structure and concentration are crucial for understanding their role in biochemistry and physiology, as well as for developing diagnostic and therapeutic strategies for purine-related diseases.
Purines are involved in various physiological processes, such as energy metabolism, cell signaling, and gene expression. Imbalances in purine metabolism can lead to the development of various diseases, including gout, Lesch-Nyhan syndrome, and certain types of cancer.
By quantifying purines in biological samples, researchers and clinicians can gain insights into the underlying mechanisms of these diseases and develop targeted interventions. Additionally, purine quantification can be used to monitor the efficacy of treatments and to assess the impact of dietary and lifestyle factors on purine metabolism.
Conclusion
The purine ring is a fundamental structure in biochemistry, with a well-defined molecular weight and structure. Purines play a crucial role in the formation of nucleic acids and are involved in various physiological processes. Analytical techniques, such as HPLC and MS, can be used to quantify purines in biological samples with high accuracy and precision, providing valuable insights into purine metabolism and its implications for human health.
References
- Determination of four different purines and their content change in seafood by high-performance liquid chromatography. Journal of Separation Science, 34(11), 1465-1471.
- Determination of purine contents in different parts of pork and beef by high performance liquid chromatography. Journal of Chromatography A, 1216(2), 305-310.
- Simultaneous quantification of purine and pyrimidine bases, nucleosides and their degradation products in bovine blood plasma by high performance liquid chromatography tandem mass spectrometry. Journal of Chromatography B, 878(28), 2855-2863.

I am Abdullah Arsalan , Completed my PhD in Biotechnology. I have 7 years of research experience. I have published 6 papers so far in the journals of international repute with an average impact factor of 4.5 and few more are in consideration. I have presented research papers in various national and international conferences. My subject area of interest is biotechnology and biochemistry with special emphasis on Protein chemistry, enzymology, immunology, biophysical techniques and molecular biology.