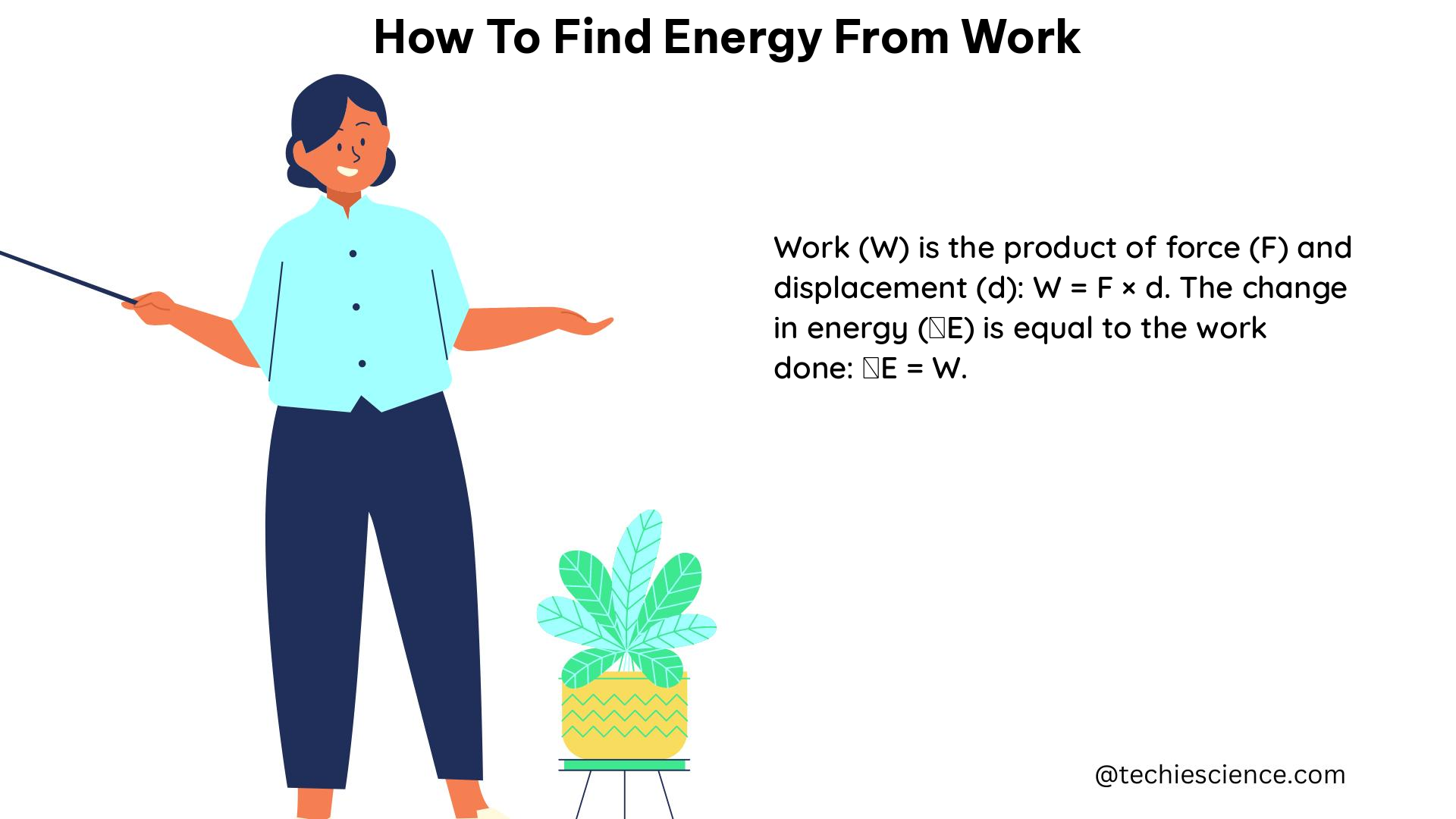
How to Calculate Gibbs Free Energy Without Temperature
Calculating Gibbs free energy (G) without temperature is not possible since it is a thermodynamic property that depends on both enthalpy (H) and entropy (S) changes at a specific temperature (T). However, if you have the standard Gibbs free energy change (ΔG°) at a specific temperature and the equilibrium constant (K) for a reaction, you … Read more