The ozone layer is a crucial component of the Earth’s atmosphere, responsible for absorbing harmful ultraviolet (UV) radiation from the sun. Understanding the density of the ozone layer is essential for studying atmospheric physics, climate change, and environmental science. In this comprehensive guide, we will delve into the intricacies of ozone layer density, providing a wealth of technical details and practical applications for physics students.
Understanding Ozone Layer Density
The ozone layer is a region in the Earth’s stratosphere where the concentration of ozone (O₃) molecules is relatively high. The density of the ozone layer is typically measured in Dobson Units (DU), which is the most common unit for quantifying ozone concentration.
One Dobson Unit represents the number of ozone molecules that would be required to create a layer of pure ozone 0.01 millimeters thick at a temperature of 0 degrees Celsius and a pressure of 1 atmosphere. Expressed mathematically, 1 DU is equivalent to 2.69 × 10^16 ozone molecules per square centimeter of the Earth’s surface.
The average ozone layer density over the Earth’s surface is approximately 300 Dobson Units, which corresponds to a layer thickness of about 3 millimeters (0.12 inches). However, the ozone layer density can vary significantly depending on location, altitude, and other factors.
Factors Affecting Ozone Layer Density
The density of the ozone layer is influenced by a variety of factors, including:
-
Latitude: The ozone layer density is typically highest in the tropics and lowest in the polar regions. This is due to the differences in solar radiation and atmospheric circulation patterns.
-
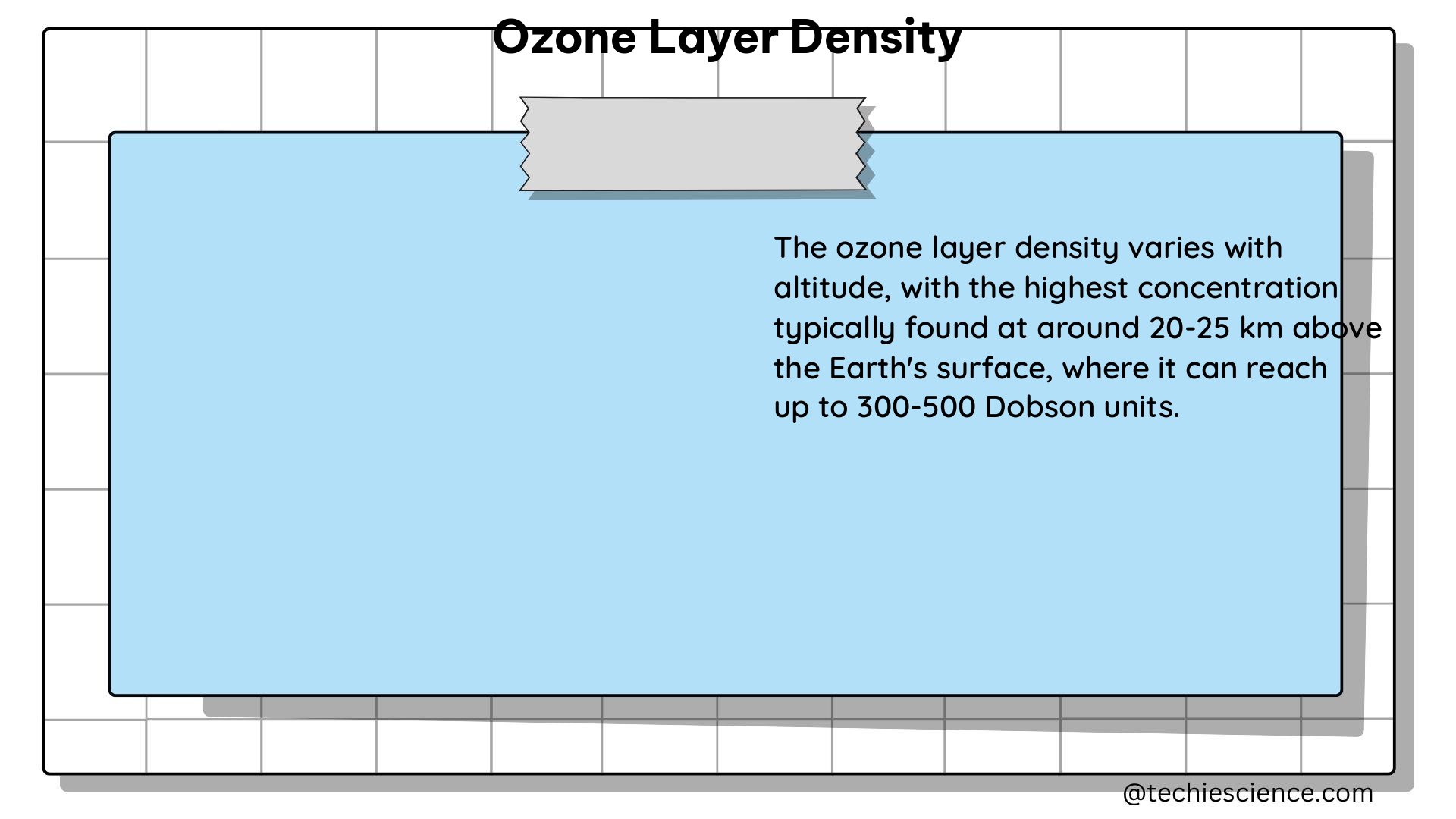
Altitude: The ozone layer is not a single layer, but rather a region in the stratosphere where ozone concentration is elevated. The peak ozone concentration is typically found at an altitude of around 20-25 kilometers above the Earth’s surface.
-
Seasonal Variations: The ozone layer density can undergo seasonal changes, with the highest concentrations observed in the spring and the lowest in the fall.
-
Ozone-Depleting Substances: Certain chemicals, such as chlorofluorocarbons (CFCs) and other halogenated compounds, can deplete the ozone layer by catalyzing the breakdown of ozone molecules. This can lead to a decrease in ozone layer density, particularly in the polar regions.
-
Solar Activity: The amount of solar radiation reaching the Earth’s atmosphere can affect the production and destruction of ozone molecules, thereby influencing the ozone layer density.
-
Meteorological Conditions: Factors like temperature, wind patterns, and atmospheric circulation can also impact the distribution and concentration of ozone in the stratosphere.
Measuring Ozone Layer Density
The density of the ozone layer is typically measured using specialized instruments and techniques, including:
-
Dobson Spectrophotometer: This instrument measures the amount of UV radiation absorbed by the ozone layer, which is then used to calculate the ozone layer density in Dobson Units.
-
Satellite Observations: Satellites equipped with remote sensing instruments, such as the Ozone Monitoring Instrument (OMI) and the Atmospheric Infrared Sounder (AIRS), can provide global measurements of ozone layer density.
-
Ozonesonde Measurements: Balloons equipped with ozone sensors can be launched to measure the vertical profile of ozone concentration in the atmosphere, which can be used to estimate the ozone layer density.
-
Ground-based Monitoring Stations: A network of ground-based monitoring stations around the world, such as the World Ozone and Ultraviolet Radiation Data Centre (WOUDC), collect data on ozone layer density and other atmospheric parameters.
Ozone Layer Depletion and Recovery
The ozone layer has faced significant challenges in recent decades due to the widespread use of ozone-depleting substances, such as CFCs. This has led to the formation of the “ozone hole” over the Antarctic region, where the ozone layer density can drop to as low as 100 Dobson Units, forming a layer only 1 millimeter thick.
In response to this threat, the Montreal Protocol was established in 1987, which has led to a gradual phase-out of ozone-depleting substances. As a result, the ozone layer is showing signs of recovery, with the potential for a full recovery by the middle of the 21st century.
Practical Applications of Ozone Layer Density
Understanding the density of the ozone layer has numerous practical applications in various fields, including:
-
UV Radiation Exposure: Knowing the ozone layer density can help predict the level of harmful UV radiation reaching the Earth’s surface, which is crucial for public health and safety.
-
Climate Change Studies: The ozone layer plays a crucial role in regulating the Earth’s temperature and climate, and changes in its density can have significant implications for global climate patterns.
-
Atmospheric Modeling: Accurate measurements of ozone layer density are essential for developing and validating atmospheric models used in weather forecasting and climate projections.
-
Satellite and Aerospace Operations: Understanding the ozone layer density is important for satellite and spacecraft operations, as it can affect the performance and lifespan of these systems.
-
Environmental Monitoring: Monitoring the ozone layer density is crucial for assessing the effectiveness of policies and regulations aimed at protecting the environment and mitigating the effects of ozone depletion.
Conclusion
The ozone layer is a vital component of the Earth’s atmosphere, and understanding its density is crucial for a wide range of scientific and practical applications. This comprehensive guide has provided a detailed overview of the technical aspects of ozone layer density, including the Dobson Unit, factors affecting its distribution, measurement techniques, and the ongoing efforts to protect and restore the ozone layer. By mastering the concepts presented here, physics students can gain a deeper understanding of atmospheric physics and contribute to the ongoing efforts to safeguard the Earth’s environment.
References
- Dobson Unit facts – NASA Ozone Watch. (2011-07-22). Retrieved from https://ozonewatch.gsfc.nasa.gov/facts/dobson_SH.html
- Scientific Assessment of Ozone Depletion 2022: Twenty Questions and Answers About the Ozone Layer. (2022). Retrieved from https://csl.noaa.gov/assessments/ozone/2022/twentyquestions/
- Ozone Watch: Latest status of ozone. (n.d.). Retrieved from https://ozonewatch.gsfc.nasa.gov
- Ozone Layer – Our World in Data. (n.d.). Retrieved from https://ourworldindata.org/ozone-layer

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.