Nitric oxide is one of the most important nitrogen oxides. It is highly noxious when absorbed through the skin and inhale. Let us see nitric oxide’s different properties.
Nitric oxide is a toxic gas that is formed while nitrogen is oxidized. Electric sparks and high temperatures are used to create nitric oxide from nitrogen and oxygen. It has a significant role in chemical signaling in both humans and other animals.
Let us see about the chemical formula, electron configurations, paramagnetic properties, and many more properties of nitric oxide in this article.
NO IUPAC name
Nitric oxide is the preferred IUPAC nomenclature. Nitric oxide is sometimes specified as nitrogen monoxide, Mononitrogen monoxide.
NO chemical formula
Nitric oxide has the chemical formula NO. A free is present in the diatomic molecule of nitric oxide, which is relatively unstable (i.e., an unpaired electron). The molecule can either gain or lose one electron in order to form the ions NO– and NO+.
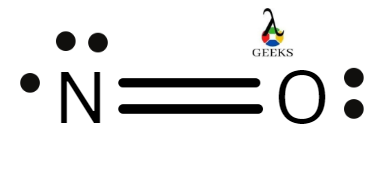
NO CAS number
The CAS number of nitric oxide is 10102-43-9.
NO ChemSpider ID
The ChemSpider ID of nitric oxide is 127983.
NO chemical classification
- Nitric oxide exists as a free radical.
- Nitric oxide is classified as a simple asphyxiant.
NO molar mass
A mole of nitric oxide has a mass of 30.01 grams.
NO color
NO is colorless due to the presence of 1 unpaired electron.
NO viscosity
Nitric oxide viscosity at 0° C temperature and 1 atm pressure is 0.0178 centipoises.
NO molar density
The molar density of nitric oxide is 0.0447mol/L since it has a density of 1.3402g/L.
NO melting point
The melting point of nitric oxide is about -164°C.
NO boiling point
Nitric oxide has a boiling point of about -152°C.
NO state at room temperature
Nitric oxide is an oxidizing gas that is non-flammable and has an offensive odor at room temperature.
NO covalent bond
Nitric oxide is a covalent non-polar compound is composed of two non-metals: nitrogen (N) and oxygen (O).
NO covalent radius
NO has no covalent bond since they have double bonds.
NO electron configurations
The electron configuration of an element describes how electrons are distributed in its atomic orbitals. Let us see the electronic configuration of NO.
The ground state electronic configuration of the nitric oxide molecule can be written as (σ1s) 2(σ1s*) 2(σ2s)2(σ2s*)2(π2px)2(π2py)2(σ2px)2(π2px*)1
NO oxidation state
Nitric oxide has a +2 oxidation state for nitrogen and a –2 oxidation state for oxygen.
NO acidity/alkaline
Nitric oxide does not show signs of basic or acidic characteristics when reacting with water. Therefore, it is neutral oxide.
Is NO odorless?
Nitric oxide has a nice odor that quickly turns brown in high concentrations in the atmosphere.
Is NO paramagnetic?
The substance’s unpaired electrons enrich it with paramagnetic properties. Let us elucidate if nitric oxide is paramagnetic or not.
Nitric oxide is paramagnetic in gaseous form. It has eleven valence electrons. However, in liquid and solid states, it exists as a symmetrical or asymmetrical dimer and is thus diamagnetic.
NO hydrates
Nitrous oxide (N2O), also known as laughing gas, exists in solid form as a hydrated form.
NO structure
Nitric oxide is made up of a single nitrogen atom bonded to an oxygen atom. The double bar (=) between the two chemical symbols represents a double bond between nitrogen and oxygen.
NO polarity and conductivity
- Nitric oxide has a net dipole moment and is a polar molecule. The dipole moment of nitric oxide, in Debye units, is 0.159.
- The thermal conductivity (Unit: Mille Watts per Meter Kelvin) of nitric oxide varies with the temperature.
| Temperature(k) | Thermal conductivity(mW/mk) |
|---|---|
| 200 | 17.8 |
| 300 | 25.9 |
| 400 | 33.1 |
| 500 | 39.6 |
| 600 | 46.2 |
NO reaction with acid
Nitrous acid and Nitrogen dioxide are created when gaseous nitric oxide reacts with nitric acid on silica surfaces in the presence of water at room temperature.
HNO3 + NO (g) →HONO(s) + NO2 (g)
NO reaction with base
Nitric oxide is a non-reactive oxide, so it shows neither basic nor acidic properties, and they do not form salts when they react with acids or bases.
NO reaction with oxide
When nitric oxide (NO) reacts with oxygen gas, nitrogen dioxide, a gas with a deep brown color, is formed.
2NO (g) + O2 (g) →2NO2 (g)
NO reaction with a metal
Metal nitrosyls are formed when nitric oxide reacts with transition metals. Cobalt tricarbonyl nitrosyl is produced as a by-product of the nitrosylation of cobalt carbonyl.
Co2 (CO) 8 + 2 NO → 2 CoNO (CO) 3 + 2 CO
Conclusion
Nitric oxide is a colorless and toxic gas. It has a strong but pleasant odor.It is a covalent non-polar compound. It is a non-reactive oxide; they do not form salts when they react with acids or bases.

Hi … I’m Saina Naushad. I completed my Masters in science with a specialization in Chemistry. I worked with advanced research techniques during my science studies and possessed deep knowledge and expertise in different chemistry topics. I want to help learners better understand advanced Chemistry concepts by sharing my knowledge and skills. please reach out to me on LinkedIn.