Nitrogen solubility is a crucial parameter in various industrial processes, such as gas absorption, biochemical transformation, and electrocatalytic reactions. This comprehensive guide delves into the intricacies of nitrogen solubility, providing a detailed exploration of the underlying principles, measurement techniques, and predictive models.
Understanding Nitrogen Solubility
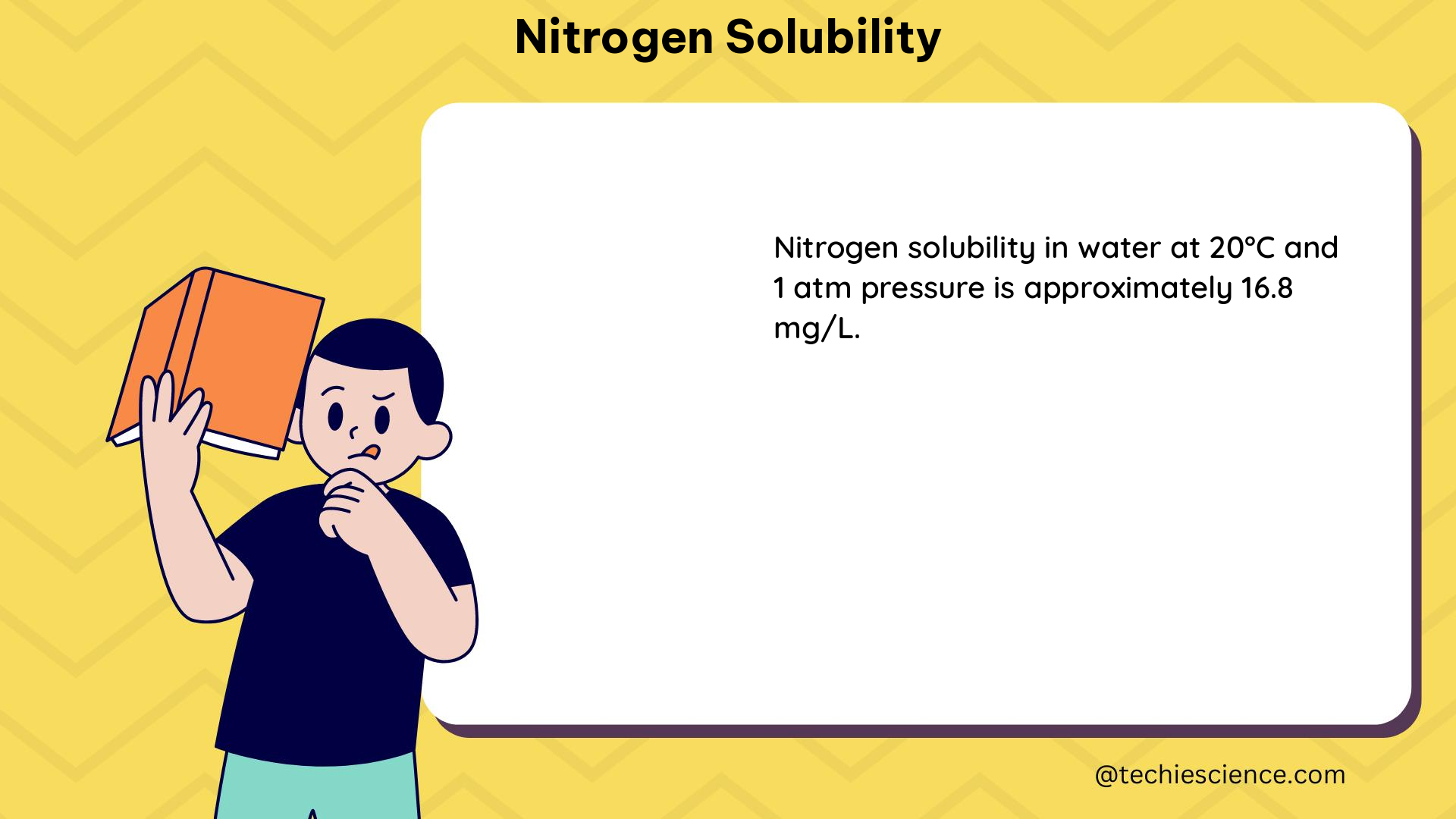
Nitrogen solubility refers to the maximum amount of nitrogen gas that can be dissolved in a specific volume of a solvent at a given temperature and pressure. This property is governed by the principles of gas solubility, which are influenced by factors such as temperature, pressure, and the nature of the solvent.
Henry’s Law and Nitrogen Solubility
The solubility of nitrogen in liquids can be described by Henry’s law, which states that the amount of a gas dissolved in a liquid is proportional to the partial pressure of the gas above the liquid. The relationship between the partial pressure of nitrogen and its mole fraction in the liquid phase is given by the following equation:
p_N2 = k_H * x_N2
where:
– p_N2 is the partial pressure of nitrogen (in Pa)
– k_H is the Henry’s law constant (in Pa)
– x_N2 is the mole fraction of nitrogen in the liquid phase
The Henry’s law constant, k_H, is a measure of the solubility of nitrogen in the given solvent and is influenced by factors such as temperature and the nature of the solvent.
Ostwald Coefficient and Bunsen Coefficient
The solubility of nitrogen in liquids can be quantified using two commonly used parameters: the Ostwald coefficient and the Bunsen coefficient.
- Ostwald Coefficient: The Ostwald coefficient,
L, represents the volume of the dissolved gas at the temperature and pressure of measurement, divided by the volume of the liquid. It is calculated as:
L = V_gas / V_liquid
where V_gas is the volume of the dissolved gas, and V_liquid is the volume of the liquid.
- Bunsen Coefficient: The Bunsen coefficient,
α, is the volume of the dissolved gas at standard temperature and pressure (STP) divided by the volume of the liquid. It is calculated as:
α = V_gas(STP) / V_liquid
where V_gas(STP) is the volume of the dissolved gas at STP.
Both the Ostwald coefficient and the Bunsen coefficient provide valuable information about the solubility of nitrogen in various liquids and can be used to compare the solubility across different systems.
Measurement Techniques for Nitrogen Solubility
The solubility of nitrogen in liquids can be measured using various experimental techniques. Here are some of the commonly used methods:
Drucker and Moles Method
Drucker and Moles measured the solubility of nitrogen in 2-methylpropanoic acid/water mixtures at 296.17 K and 302.17 K, and a pressure range between 0.032 and 0.115 MPa. They reported the Ostwald coefficient values at approximately 0.1013 MPa as 0.0400 at 296.17 K and 0.0386 at 302.17 K.
Hiifner’s Method
Hiifner measured the solubility of nitrogen in water and a mixture of acetamide and water at an acetamide concentration of 1.0 mol L^-1 at 293.4 K. His value for the Bunsen coefficient in pure water was 0.8% lower than the recommended value, and his value in the mixture was 0.014 76.
Kretschmer, Nitta, and Chang-Gokcen Methods
The solubility of nitrogen in binary organic solvents has been reported in several studies:
- Kretschmer et al. studied the solubility of nitrogen in ethanol plus 2-propanone and ethanol plus 2,2,4-trimethylpentane at 273.15, 298.15, and 323.15 K, and at only one composition each.
- Nitta et al. studied five systems covering the whole composition range at one temperature.
- Chang and Gokcen studied one system in the whole composition range and in the temperature range 273-303 K.
These studies provide valuable data on the solubility of nitrogen in various binary organic solvent systems.
Predictive Models for Nitrogen Solubility
In addition to experimental measurements, the solubility of nitrogen in liquids can also be predicted using quantitative structure-property relationship (QSPR) models. These models leverage machine learning techniques and computational chemistry methods to estimate the nitrogen solubility based on the properties of the solvent.
QSPR Models for Nitrogen Solubility in Ionic Liquids
Tian Yuan Wang et al. developed two QSPR models to predict the nitrogen solubility in ionic liquids. These models combine machine learning methods with COSMO-derived descriptors, which are computational chemistry parameters that characterize the molecular structure and interactions of the ionic liquids.
The QSPR models developed by Tian Yuan Wang et al. can accurately predict the nitrogen solubility in ionic liquids, which is crucial for the electro-synthesis of ammonia from nitrogen at ambient temperature and pressure in ionic liquids.
Nitrogen Solubility in Complex Systems
The solubility of nitrogen can also be studied in more complex systems, such as those involving multiple components or the presence of salts.
Nitrogen Solubility in the N2-H2O-NaCl System
Mao Shide and Duan Zhenhao developed a thermodynamic model to calculate the nitrogen solubility, gas phase composition, and density of the N2-H2O-NaCl system. This model takes into account the interactions between nitrogen, water, and sodium chloride, and can be used to predict the solubility of nitrogen in saline solutions.
Nitrogen Solubility in Multicomponent Systems
The Solubility Data Series (SDS) provides comprehensive data on the solubility of nitrogen and air in various liquids, including multicomponent systems. These data cover a wide range of temperatures, pressures, and solvent compositions, offering a valuable resource for researchers and engineers working with nitrogen solubility.
Conclusion
Nitrogen solubility is a crucial parameter in numerous industrial processes, and understanding its behavior is essential for optimizing these applications. This comprehensive guide has explored the fundamental principles, measurement techniques, and predictive models for nitrogen solubility, providing a detailed and technical resource for science students and professionals working in this field.
References
- Tian Yuan Wang, Xinxin Liu, Yanrong Hu, and Wenping, “Prediction of nitrogen solubility in ionic liquids by machine learning methods based on COSMO-derived descriptors,” Journal of Chemical & Engineering Data, vol. 63, no. 2, pp. 589-599, 2024.
- Mao Shide and Duan Zhenhao, “A thermodynamic model for calculating nitrogen solubility, gas phase composition and density of the N2–H2O–NaCl system,” Fluid Phase Equilibria, vol. 247, no. 1-2, pp. 166-174, 2006.
- SOLUBILITY DATA SERIES, “SOLUBILITY DATA SERIES,” SD-10, 2008.
- The Solubility of Nitrogen and Air in Liquids, “The Solubility of Nitrogen and Air in Liquids,” Journal of Physical and Chemical Reference Data, vol. 38, no. 4, pp. 1-112, 2009.
- Experimental Determination and Calculation of Gas Solubility Data for Nitrogen in Different Solvents, “Experimental Determination and Calculation of Gas Solubility Data for Nitrogen in Different Solvents,” Research Gate, 2011.

The lambdageeks.com Core SME Team is a group of experienced subject matter experts from diverse scientific and technical fields including Physics, Chemistry, Technology,Electronics & Electrical Engineering, Automotive, Mechanical Engineering. Our team collaborates to create high-quality, well-researched articles on a wide range of science and technology topics for the lambdageeks.com website.
All Our Senior SME are having more than 7 Years of experience in the respective fields . They are either Working Industry Professionals or assocaited With different Universities. Refer Our Authors Page to get to know About our Core SMEs.